Low HDL is not associated with Coronary Heart Disease in Non-diabetic Agrarian Rural Community in Central India
Citation: Ingle S, et al. Low HDL is not associated with Coronary Heart Disease in Non-diabetic Agrarian Rural Community in Central India. Ann Med Health Sci Res. 2018;8:354-359
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Background & objectives: Coronary Heart Disease (CHD) is the disease of urbanisation. However, the recent evidence suggests that CHD is becoming more prevalent in low socioeconomic group, including rural areas. The role of lipids and lipoproteins in pathogenesis of CHD factors is well established. Methods: We performed a case-control study to determine association of dyslipidaemia with CHD in individuals above 60 years residing in the rural community of central India. Successive consenting nondiabetic subjects who are diagnosed to have definite CHD (electrocardiographically defined; exercise test and echocardiographically/angiographically confirmed) but are not on statins were enrolled (cases n=41). Subjects with matched age group, gender and socioeconomic status, with no evidence of CHD, nondiabetic and not on statin, were recruited as controls (n=164). Results: Isolated low HDL level was the most common finding (80% in male, 90% in female cases and 74% in male and 95% in female controls). No statistically significant difference was found in cases and controls in total cholesterol (p=0.3342), HDLCholesterol (p=0.4864), TG (p=0.997), LDL cholesterol (p=0.1945) and VLDL cholesterol (p=0.5943) and lipid ratios i.e. total cholesterol: HDL (p=0.075), TG: HDL (0.65) and LDL: HDL (0.05). The correlation coefficient between serum HDL and waist circumference, serum HDL and BMI indicated no association. There was no difference in the distribution of S. HDL-C among subjects who belonged to different socioeconomic strata. Conclusion: Overall prevalence of lipid abnormalities is low in our population except isolated low HDL level. We found that isolated low HDL level was not associated with CHD in our rural community.
Keywords
Low HDL; Coronary heart disease; Rural
Introduction
The increasing prevalence of Coronary Heart Disease (CHD) in rural population in India is following foot-steps of urban population over last two decades. The ecological analysis of major CHD risk factors and mortality demonstrates high co-relations between expected and observed mortality rate for three main risk factors- Hypertension, serum lipids and smoking. Dyslipidaemia is considered the most important atherosclerotic risk factor. Though lower than high-income countries, population based studies in India suggest prevalence of high cholesterol as high as 25–30% of urban and 15–20% rural subjects. [1] The most common pattern of dyslipidaemia found in India is combination of borderline high LDL cholesterol, low HDL cholesterol and high triglycerides. Raised apolipoprotein B, total cholesterol, LDL cholesterol and non-HDL cholesterol have been shown to have significant association of coronary events while inverse association with high apolipoprotein A and HDL cholesterol is seen. To our knowledge there is no community based case-control study done to observe the pattern of dyslipidaemia in patients with confirmed CHD in rural India. Hence, the present community-based case control study was undertaken to find the association of dyslipidaemia in subjects with confirmed CHD who do not have diabetes and are not on any lipid lowering medications.
Methods
A case control study was performed in selected villages around Medical College Hospital (Kasturba Hospital) Sewagram in Wardha district of Maharashtra. The Kasturba Hospital had adopted villages under a holistic health care assurance programme from 1985 onwards, which provides an affordable and accessible health care at the doorstep in surrounding villages. The scheme has earned participation by entire community. This study is a part of a larger parallel studies undertaken to identify prevalence of noncommunicable diseases like CHD, Hypertension, Diabetes Mellitus, Stroke and Nephropathy in this area. The demographic data about age, sex, obesity (BMI, WC), socioeconomic status and education was available to us.
CHD cases were diagnosed on the basis of past documentation, response to WHO-Rose Angina questionnaire, changes in electrocardiogram, stress test (6-minute walk test), 2D echocardiography and coronary angiography whenever it was required. Four age, sex and socioeconomic structure -matched controls were chosen for each case using computer generated random number. Socioeconomic grade (scale developed to measure standard of living by National Family Health survey –N.F.H.S.- which judges socioeconomic status by materialistic possessions) [2] of the entire population was already available from parallel studies.
Overnight fasting samples were collected from both cases and controls, for lipid profile (total cholesterol (TC), triglycerides (TG), high density lipoproteins (HDL), low density lipoproteins (LDL), very low-density lipoproteins (VLDL) and fasting blood glucose levels. Total cholesterol was analyzed by CHOD-PAP (Cholesterol oxidases and phenol aminophenazone), Triglycerides by GPO-PAP Trinder (glycerol 3 phosphate oxidase), HDL-C by immune inhibition, VLDL-C was calculated by indirect method as VLDL cholesterol is one-fifth of triglyceride level. LDL-C was calculated by subtracting VLDL-C and HDL-C from total cholesterol. ERBA kits supplied by Transia Biochemicals Ltd., Mumbai were used. Cut off levels for lipid parameters were decided according to NCEP-ATPIII guidelines. [3] The laboratory at Kasturba Hospital runs internal quality control (IQC) twice daily to check random and systematic errors as per National Accreditation Base Lab (NABL). For external quality control (EQC), the lab is affiliated to EQC program with Christian Medical College (CMC), Vellore since 1972 and Randox (RIQAS) for the last 7 years. In both of these programs, our lab has got good rank continuously.
All the subjects found to have CHD and/or dyslipidaemia were referred to Kasturba Hospital for further evaluation and management.
Statistical analysis
Statistical data analysis was done using R version 3.4.0 (released on 21 Apr 2017). Categorical variables were summarized as frequency and percentage, while continuous variables were summarized using mean ± standard deviation (SD). Independent student t-test, i.e. Welch two sample t-test and ANOVA F-test was used for continuous variables. Odds ratio with 95% confidence interval was calculated using Mantel- Haenszel method for matched case control study. A p-value of less than 0.05 was considered to constitute a statistically significant difference.
Results
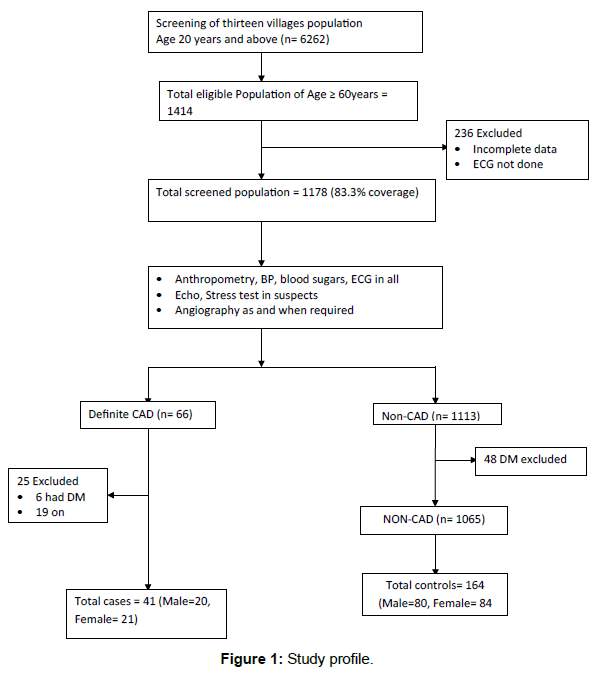
Out of 1414 individuals 60 years and above in the community, 1178 were screened (coverage=83.31%) and 66 cases were confirmed to have CHD. By excluding patients with Diabetes and patients who were already on statin therapy, 41 cases and 164 controls were selected with matching age group, sex and socioeconomic status, in the ratio of 1:4 using computer generated random number. The process for recruitment and enrolment of cases and controls is summarized in Figure 1. Table 1 shows demographic details of study population.
| Total population screened, n=1178 (%) | Cases, n=41 (%) | Control, n=164 (%) | |||
|---|---|---|---|---|---|
| Age group (yr.) | |||||
| 60-69 | 788 (66.9) | 27 (65.9) | 108 (65.9) | ||
| 70-79 | 322 (27.3) | 12 (29.3) | 48 (29.3) | ||
| ≥ 80 | 68 (5.8) | 2 (4.8) | 8 (4.8) | ||
| Sex | |||||
| Male | 572 (48.6) | 20 (48.8) | 80 (48.8) | ||
| Female | 606 (51.4) | 21 (51.2) | 84 (51.2) | ||
| Socioeconomic status | |||||
| Poor | |||||
| Grade 1 (very poor) | 118 (10) | 4 (9.8) | 8 (4.8) | ||
| Grade 2 (poor) | 281 (23.9) | 10 (24.3) | 50 (30.4) | ||
| Not poor | |||||
| Grade 3 (Low Middle class) | 593 (50.3) | 23 (56.0) | 91 (55.4) | ||
| Grade 4 (High Middle class) | 159 (13.5) | 1 (2.4) | 15 (9.1) | ||
| Grade 5 (Upper class) | 27 (2.3) | 3 (7.3) | 0 | ||
| BMI | <= 18.5 | 307 (26.1) | 13 (31.7) | 39 (23.7) | |
| 18.51- 22.99 | 632 (53.7) | 17 (41.4) | 82 (50.0) | ||
| >=23 | 239 (20.3) | 11 (26.8) | 43 (26.2) | ||
| WC | Male | <90cms | 538 (45.7) | 17 (41.5) | 77 (47) |
| ≥90cms | 34 (2.9) | 3 (7.3) | 3 (1.8) | ||
| Female | <80cms | 587 (49.8) | 17 (41.5) | 65 (39.6) | |
| ≥80cms | 19 (1.6) | 4 (9.8) | 19 (11.6) | ||
| Education | |||||
| Illiterate | 588 (49.9) | 20 (48.8) | 78 (47.6) | ||
| Schooling of 1 – 5 years | 567 (48.1) | 20 (48.8) | 81 (49.4) | ||
| Schooling of 6 years or more | 23 (2) | 1 (2.4) | 5 (3.0) | ||
Table 1: Baseline characteristics of the study population.
Table 2 shows mean and standard deviations for levels of serum lipids among cases and controls. Proportion of study subjects with normal serum levels among cases and controls were 90% and 94% for respectively for TC, 80% and 85% for TG, 90% and 95% for LDL-C, and 80% and 84% for VLDL-C. However, 80% of men and 90% of women in cases and 74% of men and 95% of women in controls had low serum HDL level as per the NCEP-ATPIII criteria. While there was not even a single case of isolated high levels of TC, TG, LDL-C or VLDL-C, we found that 61% of cases and 66% of controls had isolated low HDL-C.
| Cut-off values | Cases (n=41) n (%) |
Controls (n=164) (%) |
Mean ± SD (range) for biochemical parameters | t-test value | p-value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Cases | Controls | ||||||||
| Cholesterol | <200 | 37 (90) | 155 (94) | 156.5 ± 32.9 (104-255) |
151.1 ± 27.7 (83-236) |
0.974 | 0.334 | ||
| ≥ 200 | 4 (10) | 9 (05) | |||||||
| HDL-C | Male | >40 | 4 (20) | 21 (26) | 36.3 ± 8.96 (23-69) |
37.3 ± 7.06 (21-63) |
0.701 | 0.486 | |
| ≤ 40 | 16 (80) | 59 (74) | |||||||
| Female | >50 | 2 (1) | 4 (05) | ||||||
| ≤ 50 | 19 (9) | 80 (95) | |||||||
| TG | <150 | 33 (80) | 140 (85) | 118.5 ± 56.8 (52-319) |
118.5 ± 45.4 (56-323) |
0.003 | 0.997 | ||
| ≥ 150 | 8 (20) | 24 (14) | |||||||
| LDL-C | <130 | 37 (90) | 156 (95) | 95.7 ± 22.58 (49-157.4) |
89.9 ± 23.3 (35-160.2) |
1.312 | 0.194 | ||
| ≥ 130 | 4 (10) | 8 (05) | |||||||
| VLDL-C | <30 | 33 (80) | 136 (83) | 23.6 ± 11.3 (10.4-63.8) |
24.7 ± 13.2 (11.2-64.6) |
0.535 | 0.594 | ||
| ≥30 | 8 (20) | 28 (17) | |||||||
| TC: HDL | 4-4.5 | 4.51 ± 1.24 | 4.13 ± 0.86 | 1.81 | 0.075 | ||||
| TG: HDL | 3.8 | 3.52 ± 2.05 | 3.36 ± 1.70 | 0.44 | 0.65 | ||||
| LDL: HDL | 2.5-3 | 2.79 ± 0.97 | 2.46 ± 0.69 | 2.00 | 0.05 | ||||
| Isolated low HDL | Male | ≤ 40 | 13 (65) | 51 (65) | 36.09 ± 5.68 | 35.00 ± 6.31 | 0.85 | 0.4 | |
| Female | ≤ 50 | 12 (57) | 57 (67) | ||||||
Table 2: Distribution of lipids around cut off values with analysis in cases and controls.
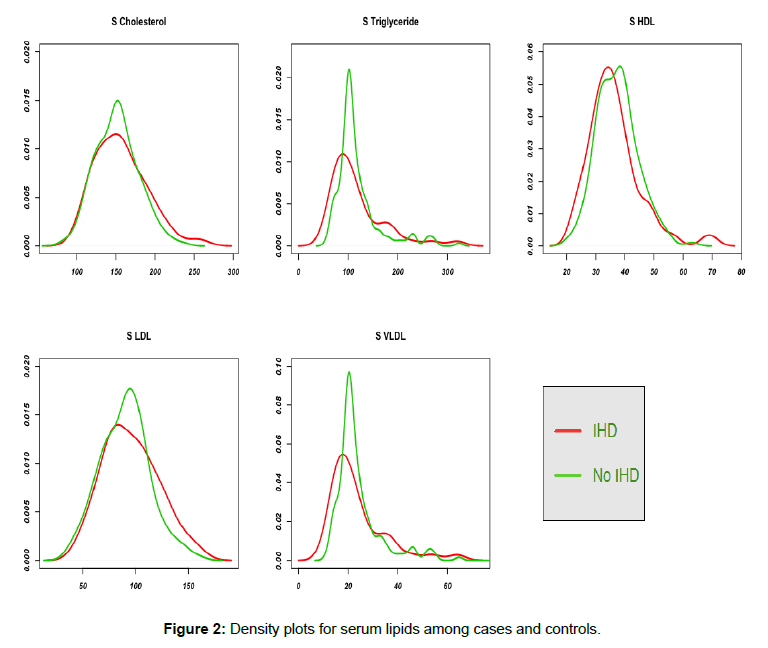
We did not find significant difference in the mean levels of serum lipids (i.e. TC, TG, LDL, VLDL and HDL) between cases and controls. LDL to HDL ratio and total cholesterol to HDL ratio were 2.79±0.97 and 4.51±1.24 respectively among cases compared to 2.46±0.69 and 4.13±0.86 among controls (p-value of 0.05 and 0.075 respectively). Isolated low HDL-C did not differ statistically in cases and controls (p-value 0.4). Density plots of the serum lipids in Figure 2 vividly demonstrate the same.
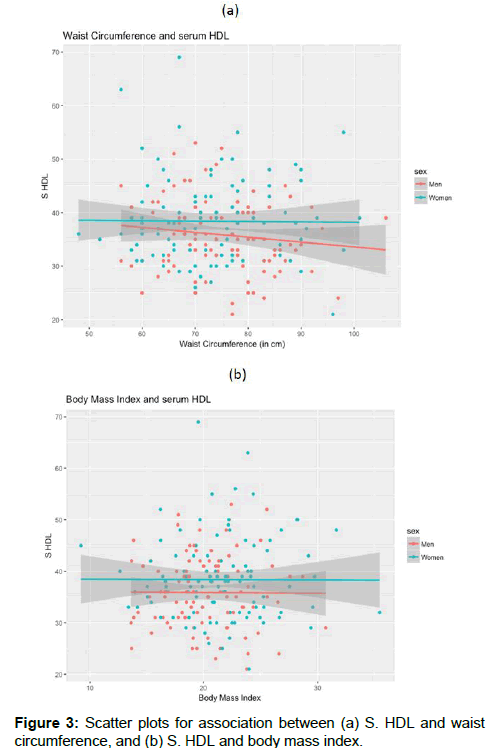
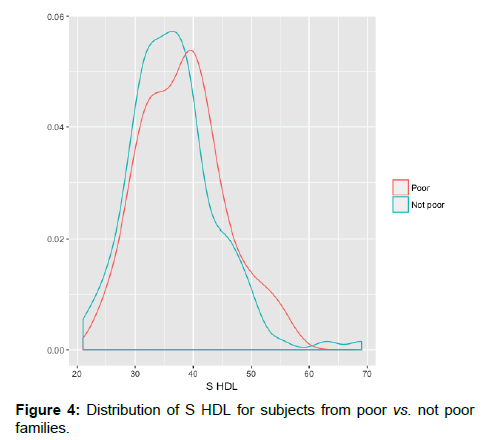
The overall prevalence of overweight or obese people was low (20.3%). We calculated Pearson correlation coefficient separately for men and women to understand the association of serum HDL with waist circumference and body mass index. The correlation coefficient between serum HDL-C and waist circumference was -0.01 for women and -0.12 for men and that between serum HDL-C and BMI was -0.004 for women and -0.007 for men, which indicated no association of S. HDL with WC or BMI [Figure 3]. There was no difference in HDL-C among subjects who belonged to poor families when compared with those from not poor families [Figure 4].
Discussion
This was a rural community based case-control study to observe distribution of lipid parameters among confirmed CHD cases. 34.1% and 35.2% in cases and controls of our study population respectively belonged to poor socio-economic group and 56% and 55.4% to lower middle socio-economic group. To our understanding there is no other study conducted in India for evaluation of CHD in similar population.
Though dyslipidaemia is a well-established risk factor for CHD, we did not observe any lipid parameter (high total cholesterol, triglycerides, low density lipoproteins, low levels of high density lipoproteins, TC/ HDL, TG/HDL, LDL/HDL ratios) associated with higher risk of CHD in present study. In our study WC, BMI and socioeconomic status also did not co-relate with lipid levels in CHD cases or controls.
Interestingly, isolated low HDL-C levels though highly prevalent in both cases (61%) as well as controls (66%) did not differ statistically in CHD cases and controls. There was no difference in distribution of serum HDL-C with other CHD risk factors like waist circumference or BMI.
Prevalence of low levels of HDL-C in Asians documented though various studies differs in urban/rural locations, with race and ethnicity [4-14] [Table 3]. These studies document simulltaneous rise in other lipid components TC, TG and LDL-C along with lowering of HDL-C levels. Normal levels of triglyceride and low HDL (TG paradox) is seen in insulin-resistant African Americans, West Africans, and black South Africans. [15-17] We report here low HDL-C levels in non-diabetic CHD cases and non-diabetic, age, sex, socioeconomic grade matched control rural polulation, unaccompanied by rise in TC, TG and LDL-C. We are not aware of any study that documents CHD cases with low HDL-C without significant rise in other lipid components.
| Sr.no | Studies | Urban/Rural | Type of study | Study population | Low HDL | Other lipids | ||
|---|---|---|---|---|---|---|---|---|
| Male | Female | |||||||
| Chaddha et al. | U+R | Community based epidemiology study | 25-64 y U= 13723 R=3375 | 2.2 (U) 8 (R) |
1.6 (U) 3.5 (R) |
Raised TC, TG, LDL | ||
| Gupta et al. | U+R | Cohort study of male population | M= 3397 (age 20-73 y) U=199, R=202 |
23.9% | Raised TC, TG, LDL | |||
| Gupta et al. | U | Epidemiological study | In 1995- 2212 subjects | 43.2 | 45.9 | Raised TC, TG, LDL | ||
| In 2002- 1123 subjects | 54.9 | 54.2 | ||||||
| Patel et al. | U+R | Population based survey | 5305 (age≥ 35 y) |
24% (U) 44% (R) |
Raised TG | |||
| Misra et al. | U | Epidemiological population study | Total 2050 subjects (883 male, 1167 female) age≥ 18 y | 39.2% | 80.3% | Raised TC, TG | ||
| Ramchandran et al. | U | Cross sectional study | N=2640 Age 12-19 y | 57% | Raised TG | |||
| Deepa et al. | U | Epidemiological study | 2650 (age =27-53 y) | 55.4 | 70.6 | Raised TG | ||
| Prabhakaran et al. | U | Cross sectional study | 2859 (M=1394, F=1465) | 59.8 | 77.7 | Raised TC, TG | ||
| R | 1185 (M=548, F=637) | 53.3 | 78.2 | |||||
| Kinra et al. | R | Cross sectional study | 1983 (age 20-69 y) | 48.5% | Raised TG | |||
| Kanjilal et al. | U+R | Epidemiological study | 2316 (age >= 18 years) M=1355 F=961 |
65.5 % | Raised TG | |||
| Joshi et al. | R+U 4 states |
Cross sectional study | 20-90 y, 14,277 (coverage 86%) |
76.8% in Jharkhand, 68.9% Tamil Nadu, no rural urban difference |
Raised TC, TG, LDL | |||
| Gupta et al. | U | Cross sectional study | 6198 (20-75 y) M-3426 F-2772 |
Northern states | 34.4% | 56.5% | Raised TC, TG | |
| Southern states | 41.1% | 64.2% | ||||||
Table 3: Asian studies with low HDL.
Despite regional differences, low HDL-C was most common lipid abnormality in the ICMR –INDIA B study [18] in all four regions (South – Tamil Nadu, West- Maharashtra, East – Jharkhand and North- Chandigarh) and no rural urban difference was observed. In Chennai urban population, though mean HDL-C was low, there was no difference in mean HDL-C levels between the group with CHD (ECG defined) and group without CHD. [19] This appears to be part of Asian Indian phenotype [20] which includes increased plasma insulin levels, insulin resistance, increased waist circumference, excess visceral fat and low adiponectin levels. Even in a Japanese population Sone et al. [21] noted that HDL-C was not a significant risk factor for CHD. Chadha et al. noticed significantly low HDL-C and high TG but low LDL-C levels in rural areas in comparison to urban. Mean levels of total cholesterol and LDL-C were higher among urban subjects. The prevalence of ECG defined CHD was high (96.7 per 1000) than in rural population (27.1%). Inspite of higher percentage of low HDL-C levels and high TG in rural area, prevalence of CHD was lower. [4] It is also proposed that low HDL-C level may not enhance risk of CHD but rather represents a marker for increased level of other atherogenic lipoproteins particularly LDL-C which was also not true in our study as the other lipoprotein parameters were also not significantly different.
Elevated triglyceride level is one of the leading cause of depressed HDL-C levels. [22,23] Commonly prescribed drugs also may lower HDL-C levels like diuretics, progestins, androgens and beta adrenergic blocking agents without intrinsic sympathomimetic activity. [24] Certain types of fats, called trans-fatty acids, have shown to lower HDL-C levels. [25] Cigarette smoking has a “dose dependent” negative effect on HDL-C. [26] Overweight and obesity, diabetes and physical inactivity, which are associated with insulin resistance have low HDL-C. [27] Finally there are rare familial dyslipoproteinemias that result in low HDL-C levels. [28] In absence of all enumerated factors above, our observations indicate that we need to probe genetic factor for universal low HDL-C values in our rural community.
Conclusion
Summarizing, we neither found low HDL-C was not associated with CHD in nondiabetic agrarian rural community in central India, nor could correlate its distribution with WC, BMI and socioeconomic grade. Our study represents predominantly poor, non-obese, less educated population who survive on hard physical labour. In view of absence of the traditional risk factors, the author suggests need to probe other factors like genetics and influence of agricultural chemicals including pesticides in the pathophysiology of CHD in this population.
Conflict of Interest
The authors disclose that they have no conflicts of interest.
REFERENCES
- Gupta RRR, Misra A, Sharma SK. Recent trends in epidemiology of dyslipidemias in India. Indian heart journal. 2017;69:382-392.
- Sciences IIfP. India National Family Health Survey (NFHS-3), 2005-06: International Institute for Population Sciences; 2007.
- Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Circulation. 2002;106:3143-3421.
- Chadha S, Gopinath N, Shekhawat S. Urban-rural differences in the prevalence of coronary heart disease and its risk factors in Delhi. Bulletin of the World Health Organization, Geneva. 1997;75:31.
- Gupta R, Prakash H, Kaul V. Cholesterol lipoproteins, triglycerides, rural-urban differences and prevalence of dyslipidaemia among males in Rajasthan. The Journal of the Association of Physicians of India. 1997;45:275-279.
- Gupta R, Gupta V, Sarna M, Prakash H, Rastogi S, Gupta K. Serial epidemiological surveys in an urban Indian population demonstrate increasing coronary risk factors among the lower socioeconomic strata. Journal-Association of Physicians of India. 2003;51:470-478.
- Patel A, Woodward M, Stolk R, Suriyawongpaisal P, Neal B. Serum lipid levels and the prevalence of dyslipidaemia among rural and urban Thai adults-Are the NCEP III Guidelines Appropriate? Journal-Medical Association of Thailand. 2005;88:1242.
- Misra A, Vikram N, Gupta R, Pandey R, Wasir J, Gupta V. Waist circumference cutoff points and action levels for Asian Indians for identification of abdominal obesity. International Journal of Obesity. 2006;30:106.
- Ramachandran A, Snehalatha C, Yamuna A, Murugesan N, Narayan KV. Insulin resistance and clustering of cardiometabolic risk factors in urban teenagers in southern India. Diabetes care. 2007;30:1828-1833.
- Deepa M, Farooq S, Datta M, Deepa R, Mohan V. Prevalence of metabolic syndrome using WHO, ATPIII and IDF definitions in Asian Indians: the Chennai Urban Rural Epidemiology Study (CURESâ€Â34). Diabetes/metabolism research and reviews. 2007;23:127-134.
- Prabhakaran D, Chaturvedi V, Shah P, Manhapra A, Jeemon P, Shah B, et al. Differences in the prevalence of metabolic syndrome in urban and rural India: a problem of urbanization. Chronic Illness. 2007;3:8-19.
- Kinra S BLea. Socio-demographic patterning of non-communicable disease risk factors in rural India: a cross sectional study. Bmj. 2010;341:4974.
- Kanjilal S, Shanker J, Rao VS, Khadrinarasimhaih NB, Mukherjee M, Iyengar SS, et al. Prevalence and component analysis of metabolic syndrome: an Indian atherosclerosis research study perspective. Vascular health and risk management. 2008;4:189.
- Gupta R, Sharma KK, Gupta BK, Gupta A, Saboo B, Maheshwari A, et al. Geographic epidemiology of cardiometabolic risk factors in middle class urban residents in India: A cross–sectional study. Journal of global health. 2015;5.
- Sumner AE, Zhou J, Doumatey A, Imoisili OE, Amoah A, Acheampong J, et al. Low HDL-cholesterol with normal triglyceride levels is the most common lipid pattern in West Africans and African Americans with metabolic syndrome: implications for cardiovascular disease prevention. CVD prevention and control. 2010;5:75-80.
- Sumner AE, Cowie CC. Ethnic differences in the ability of triglyceride levels to identify insulin resistance. Atherosclerosis. 2008;196:696-703.
- Goedecke JH, Utzschneider K, Faulenbach MV, Rizzo M, Berneis K, Spinas GA, et al. Ethnic differences in serum lipoproteins and their determinants in South African women. Metabolism-Clinical and Experimental. 2010;59:1341-1350.
- Joshi SR, Anjana RM, Deepa M, Pradeepa R, Bhansali A, Dhandania VK, et al. Prevalence of dyslipidemia in urban and rural India: the ICMR–INDIAB study. PloS one. 2014;9:e96808.
- Anoop Misra LK. Obesity and the metabolic syndrome in developing countries. J Clin Endocrin Metab. 2008;93:59-530.
- Deepa R, Sandeep S,Mohan V. Abdominal obesity, visceral fat and Type 2 Diabetes- "Asian Indian Phenotype". In: Mohan V, Gundu HR Rao editors. Type 2 diabetes in South Asians : Epidemiology, risk factors and prevention. New Delhi: Jaypee Brothers Medical Publishers 2006:138-152.
- Sone H, Mizuno S, Fujii H, Yoshimura Y, Yamasaki Y, Ishibashi S, et al. Is the diagnosis of metabolic syndrome useful for predicting cardiovascular disease in Asian diabetic patients? Diabetes care. 2005;28:1463-1471.
- NJ Stone CB. Mnagement of lipids in clinical practice. Caddo, Okla Professional Communications Inc. 1997.
- Conference NC. Triglyceride, high density lipoprotein, and coronary heart disease. Jama. 1993;269:505-510.
- Wallace RB, Hunninghake DB, Reiland S, Barrett-Connor E, Mackenthun A, Hoover J, et al. Alterations of plasma high-density lipoprotein cholesterol levels associated with consumption of selected medications. The Lipid Research Clinics Program Prevalence Study. Circulation. 1980;62:IV77-IV82.
- Mensink RP, Katan MB. Effect of dietary trans fatty acids on high-density and low-density lipoprotein cholesterol levels in healthy subjects. New England Journal of Medicine. 1990;323:439-445.
- Criqui MH, Wallace RB, Heiss G, Mishkel M, Schonfeld G, Jones G. Cigarette smoking and plasma high-density lipoprotein cholesterol. The Lipid Research Clinics Program Prevalence Study. Circulation. 1980;62:IV70-IV76.
- Bethesda M. National Institutes of Health: Third Report pf the National Cholestrol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). National Institutes of Health 2001.
- Funke H. Genetic determinants of high density lipoprotein levels. Current Opinion in Lipidology. 1997;8:189-196.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.