Maternal and Fetal Acid-Base Chemistry: A Major Determinant of Perinatal Outcome
- *Corresponding Author:
- Dr. Omo-Aghoja L
Department of Obstetrics & Gynecology, Faculty of Clinical Medicine, Delta State University, Abraka, Nigeria.
E-mail: eguono_2000@yahoo.com
Citation: Omo-Aghoja L. Maternal and fetal acid-base chemistry: A major determinant of perinatal outcome. Ann Med Health Sci Res 2014;4:8-17.
Abstract
Very small changes in pH may significantly affect the function of various fetal organ systems, such as the central nervous system, and the cardiovascular system with associated fetal distress and poor Apgar score. Review of existing data on maternal-fetal acid-base balance in pregnancy highlight the factors that are associated with derangements of the acid-base status and the impact of the derangements on fetal outcome. Extensive search of electronic databases and manual search of journals for relevant literature on maternal and fetal acid chemistry, clinical studies and case studies were undertaken. There is a substantial reduction in the partial pressure of carbon dioxide (pCO2) in pregnancy. Adequate buffering prevents significant changes in maternal arterial pH. Normal fetal metabolism results in the production of acids which are buffered to maintain extracellular pH within a critical range. Fetal hypoxia can occur when maternal oxygenation is compromised, maternal perfusion of the placenta is reduced, or delivery of oxygenated blood from the placenta to the fetus is impeded. When adequate fetal oxygenation does not occur, metabolisms proceed along with an anaerobic pathway with production of organic acids, such as lactic acid. Accumulation of lactic acid can deplete the buffer system and result in metabolic acidosis with associated low fetal pH, fetal distress and poor Apgar score. There is a significant reduction in pCO2 in pregnancy. This change, however, does not result in a corresponding significant reduction in maternal arterial pH, because of adequate buffering. Very small changes in pH may cause significant derangement in fetal function and outcome.
Keywords:
Acid-base balance, Determinants, Maternal and fetal, Perinatal outcome, Pregnancy
Introduction
Available evidence indicates that pregnancy is associated with a very substantial reduction in the maternal partial pressure of carbon dioxide (pCO2).[1,2] The diffusion of blood gases between the fetal and maternal circulations is probably thought to be due to this maternal change. However, because of the adequate buffering that is occurring within the blood system, there is relatively marginal change in maternal arterial pH. The consequence of this chronic reduction in pCO2 is a state of compensated respiratory alkalosis with enhanced renal excretion and therefore, decreased circulating concentrations of bicarbonate. The circulatory changes may possibly include intracellular acidosis in the erythrocytes, in which the predominant buffer is hemoglobin. This finding may signify that there is a greater potential for oxygen-hemoglobin dissociation during pregnancy, thus facilitating oxygen delivery across the placenta.[1] Hyperventilation is a primary factor in the alteration in maternal acid-base balance in pregnancy.[3]
Existing data suggests that normal fetal metabolism results in the production of acids (carbonic and organic), and despite this, the extracellular pH is kept within a critical range due to the efficient buffer mechanisms of the blood system. This is because the functions of the various fetal organ systems, such as the central nervous system and the cardiovascular system may be significantly affected by very small changes in pH.[4] The major buffers utilized by the fetus for neutralizing hydrogen ion production are plasma bicarbonate and hemoglobin. Inorganic phosphates and erythrocyte bicarbonate are also potential buffers, although they play a lesser role in fetal acid-base hemostasis.[1]
Fetal hypoxia can occur when maternal oxygenation is compromised, maternal perfusion of the placenta is reduced, or delivery of oxygenated blood from the placenta to the fetus is impeded.[1] When adequate fetal oxygenation does not occur, complete oxidative metabolism of carbohydrates to carbon dioxide (CO2) and water is impaired and metabolism proceeds along an anaerobic pathway with production of organic acids, such as lactic acid, which are not readily excreted or metabolized. Accumulation of lactic acid can deplete the buffer system and result in metabolic acidosis.[1] Maternal acidosis is associated with lower fetal pH and poor Apgar score.[4-7]
The Apgar score is the very first test given to the newborn, immediately after the birth of the baby in the delivery or birthing suite.[8,9] The test was designed to quickly evaluate a newborn’s physical condition and to determine any immediate need for extra medical or emergency care. The Apgar score is usually assessed twice: Once at 1 min after birth and again at 5 min after birth. Sometimes, if there are concerns about the baby’s condition or the score at 5 min is low, the test may be scored for a 3rd time at 10 min after birth.[8,9] Five criteria are used to evaluate the baby’s condition and each criterion is scored on a scale of 0-2, with 2 being the best score. The Apgar scoring is summarized in Table 1.
| Criteria | Score of 0 | Score of 1 | Score of 2 | Component |
|---|---|---|---|---|
| of acronym | ||||
| Skin color/ | Blue or | Blue at | No cyanosis, | Appearance |
| complexion | pale all | extremities, | body and | |
| over | body pink | extremities | ||
| (acrocyanosis) pink | ||||
| Pulse rate | Absent | <100 | =100 | Pulse |
| Reflex | No | Grimace/ | Cry or pull | Grimace |
| irritability | response | feeble | away when | |
| to | cry when | stimulated | ||
| stimulation | stimulated | |||
| Muscle | None | Some flexion | Flexed arms | Activity |
| tone | and legs | |||
| that resist | ||||
| extension | ||||
| Breathing | Absent | Weak, | Strong, lusty | Respiration |
| irregular, | cry | |||
| gasping | ||||
Table 1: The APGAR scoring system.
Newborns with scores of 7 and above are generally normal. A score of 4-6 is considered fairly low and 3 and below are generally regarded as critically low.[8,9]
The fetus produces both volatile carbonic acid (H2CO3) and non-volatile acids (non-carbonic or organic acids). The fetus produces H2CO3 during oxidative metabolism (aerobic glycolysis). Since H2CO3 is formed primarily from CO2 via hydration in the presence of erythrocyte carbonic anhydrase, the formation of H2CO3 is equivalent to CO2 generation. The rate of CO2 production, in turn, is equivalent to fetal oxygen consumption.[1,2] The non-carbonic (organic) acids in the fetus include uric acid (from the metabolism of non-sulfurcontaining amino acids), lactate, and keto acids (from the metabolism of carbohydrates and fatty acids). These noncarbonic acids are eliminated through the maternal kidneys, after diffusing slowly across the placenta. The maternal kidney regenerates bicarbonate from the excretion of the non-carbonic acids.[10]
Materials and Methods
The articles used for this review covered the period of 1953 to 2012, and in all 127 articles were retrieved following extensive literature search and of these, 65 were adapted for this article. Others were excluded either because they were extremely old publications, the full texts of the articles were not retrievable or unavailable, or where the articles were case reports. Most of the papers adapted for this review article were randomized controlled studies, cohort studies, case control studies, clinical studies, case studies and cross sectional studies. The articles were gotten following extensive literature search using the following search engines or databases: Medline, Elsevier, Medscape, eMedicine, google and PubMed. The literature search was done using the following keywords as a guide: Maternal and fetal, acid-base balance, pregnancy, determinants and perinatal outcome. Additionally, challenges of diagnosis and treatment, fetal hypoxia were also utilized. Literature on the subject was also researched using manual library search from relevant cited textbooks and articles in journals. The key and relevant aspects of maternal acid-base balance in pregnancy, the correlation between the maternal acid-base status, fetal clinical status and Apgar score were explored. Additionally, relevant aspects of fetal acid-base balance and the correlation between the fetal acid-base status and fetal clinical status were also reviewed. Also reviewed and highlighted were the challenges of diagnosis and treatment of fetal hypoxia.
Maternal acid-base balance in pregnancy
Background
During pregnancy the respiratory component of the maternal acid-base system shows a progressive fall in arterial CO2 tension and a rise in oxygen tension and in the metabolic element, there is a parallel reduction in plasma bicarbonate.[2,11] This results in a primary respiratory alkalosis and a secondary and compensatory metabolic acidosis and thus normal pH, usually on the alkaline side of the normal range. There is no true “acidosis of pregnancy” but a reduction of total acid and total base; the primary fall in the pCO2 is buffered by the action of the renal mechanism, which controls loss or conservation of bicarbonate.[2,11]
The progressive fall in the pCO2 of maternal capillary blood during pregnancy reaches a nadir at a mean level of 31.3 mmHg and an increase in base deficit to −3.5 mEq/l with a fall in standard bicarbonate to 21.3 mEq/l by the last trimester; the pH maintains an even level.[2,11]
This lowering of maternal CO2 tension increases its transplacental gradient to about 8-10 mmHg enabling it to be more readily transported from the fetus. The fetus develops, therefore, in an environment of CO2 tension similar to or even slightly lower than that of the adult.[2,11]
In labor, there is an increase in metabolic acidosis, with a base deficit of −4.8 mEq/l and a standard bicarbonate of 20.3 mEq/l, due to maternal fixed acids from uteroplacental metabolism and from ketone bodies which appear with stress; in fetal acidosis the umbilical artery has been shown to contain high levels of lactic and pyruvic acids.[12-16] Huckabee et al.[17] (1962) stated that the placenta and mother serve as large hydrogen depositories for the fetus and help it to withstand anaerobic metabolism. Whilst, however, CO2 molecules diffuse readily through the placenta, there is experimental evidence [18] of varying degree of permeability to bicarbonate ions and hence some fetal independence in regard to bicarbonate concentration. Any antacid action of substances with a high degree of dissociation, like sodium bicarbonate, or of low lipid solubility and poor placental penetration, like tromethamine, is likely to be of benefit in lowering a maternal metabolic acidosis.
The change in the dynamics of respiration in pregnancy as manifested by hyperventilation and the accompanying progressive fall in alveolar pCO2 accounts for the respiratory alkalosis of pregnancy. This fall is found to reach a mean level of 30-32 mmHg and is associated with a 21% increase in oxygen uptake. The physiological hyperventilation of pregnancy is due to the hormonal effect of progesterone on the respiratory center.[11]
In labor, the mother may exhibit forced hyperventilation and similarly under anesthesia for surgery the lungs may be excessively inflated which is akin to forced hyperventilation; both situations can cause fetal acidosis and depression of the new born.[4,11,16,19,20] However, it should be noted that moderate hyperventilation could be beneficial as it can improve fetomaternal gradients at placental sites, but may rapidly become associated with adverse maternal and fetal consequences as it gets more pronounced. Usually when a maternal pCO2 of 15 mmHg and pH of 7.650 is approached there is a fall in fetal blood oxygen saturation; maternal muscle cramps and fetal heart irregularities in rate and rhythm appear. Experimental studies in animals have shown that excessive hyperventilation causes spasm of placental vessels and slowing of the umbilical circulation with fetal acidosis.[19]
There have been inconsistent results following attempts to oxygenate the fetus in labor by giving the mother 100% oxygen to breathe.[11] It has been demonstrated that in cases of obstruction to uteroplacental flow and fetal circulation,
Correlation between the maternal acid-base status, fetal clinical status and Apgar score
Certain pregnancies are considered high-risk cases if there are maternal conditions that put the fetus, the mother or both at an increased risk of perinatal and maternal morbidity and mortality. Some clinical parameters have been used as criteria to separate pregnant women into normal and high-risk groups. [23] Some of the leading conditions based on which pregnancies are categorized as high risk are presented in Table 2 and these listed conditions are the indications for elective fetal blood sampling (FBS) in labor.[24] Additionally, it should be noted that there are also clinical parameters for which fetuses of normal pregnancies are subjected to FBS in labor and these are equally presented in Table 2. Biochemical monitoring revealed that during labor all patients showed a gradual fall in fetal pH and an increase in pCO2 and base deficit, especially in the second stage of labor and during delivery.[16] These biochemical changes were more marked in high-risk fetuses. At birth and during the 1st h of life the neonates of high-risk mothers were more acidotic and recovered more slowly than neonates of normal mothers. It therefore implies that the fetuses of high-risk pregnancies are less able to tolerate the stress of labor as compared to the fetuses of normal pregnancies. Hence biochemical and biophysical surveillance of the high-risk fetus may provide early warning of the impending difficulties so that during labor timely intervention may be instituted.[25,26]
| Categories |
|---|
| High-risk cases |
| Pre-eclampsia/eclampsia syndrome |
| Uterine inertia |
| Elderly primigravida |
| Postmaturity |
| Prolonged second stage |
| Diabetes mellitus |
| Fetal growth restriction |
| Normal |
| Clinical fetal distress |
| Meconium alone |
| Fetal tachycardia |
| Meconium and abnormal fetal heart rate |
Table 2: Indications for fetal blood sampling
Overall, maternal acidosis is associated with lower fetal pH and poor Apgar score. The newborns that had low Apgar scores were significantly more acidotic during labor and delivery and during the 1st h of life than babies born with high Apgar scores.[24,26,27]
Apart from the scenarios mentioned above that cause derangement in fetal acid-base status, other predisposing conditions that may result in this are either acute or chronic clinical conditions of the mother, placental and/or fetus.[4] Acute maternal conditions may include any clinical situation that causes hypotension or hypovolaemia such as hemorrhage, a vasovagal attack, or epidural anesthesia, with a resultant reduction of maternal blood supply and therefore oxygen delivery to the uterus. Uterine contractions can also interrupt uterine blood fl ow by a pressure rise and if prolonged, as in hypertonus, may cause hypoxia and acidosis.[4] Acute placental factors on the other hand, include conditions such as abruption that usually result in disruption of the utero-placental circulation by separating and tearing the uterine spiral arteries from the placenta.[4] Similarly, the blood fl ow from the placenta to the fetus is often affected during labor and delivery by the umbilical cord compression; this can sometimes happen before labor if there is reduced liquor or a true knot in the cord. Animal experiments have shown that there is significant reserve because the fetus can compensate by increased oxygen extraction,[28] meaning blood fl ow to the fetus must be reduced by at least 50% to cause hypoxia.[29]
Chronic clinical conditions that have been associated with fetal acid-base derangement on the other hand may also be maternal, placental or fetal.[4] Maternal causes of chronic fetal acidosis include reduced oxygenation of maternal blood, such as in severe respiratory or cardiac disease, or reduced blood flow to the placenta, as in connective tissue diseases – for example, systemic lupus erythematosus – and preeclampsia.[4] Chronic placental factors usually result from impairment of placental transfer of oxygen.[30] This is thought to result from inadequate trophoblast invasion of the myometrium in early pregnancy,[31] leading to reduced perfusion of the intervillous spaces. In animal experiments, like acute cord compression studies, utero-placental blood fl ow also needs to be reduced by at least 50% to produce fetal hypoxia.[32] This indicates that the reduction in placental transfer seen in human fetal growth restriction must be substantial to produce the hypoxia and acidosis found at cordocentesis in such cases. Finally, even with normal placental function, chronic conditions within the fetus can cause acidosis.[4] Anemia from rhesus disease, parvovirus infection, thalassaemia or feto-maternal hemorrhage, when severe enough to reduce fetal hemoglobin concentrations below 40 g/l (equivalent to an oxygen content below 2 mmol/l), can lead to a fall in pH.[33,34] Arterio-venous shunting in fetal tumors, serious cardiac structural abnormalities, or arrhythmias are other conditions, which can lead to chronic acidosis by decreased oxygenation as a result of reduced fetoplacental blood fl ow.[4]
Fetal acid-base balance
Background
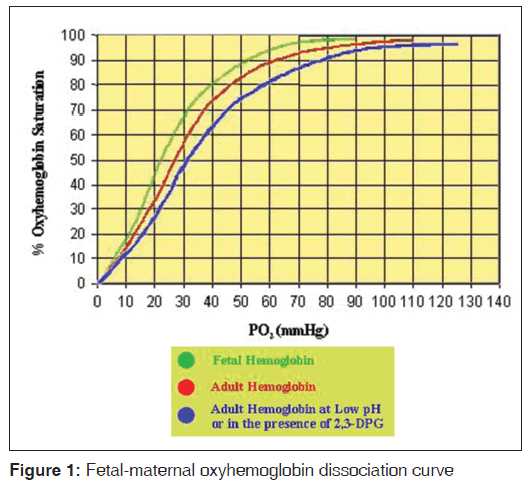
Hypoxia in fetuses is potentially very hazardous with an adverse outcome for the neonate because the fetus normally has a very low PaO2 (approximately 35 mmHg in the umbilical vein) and high PaCO2 (approximately 42 mmHg in the umbilical vein).[35] The inherent response therefore is preferential redistribution of blood to vital organs, and hence in acute severe fetal hypoxemia there is associated and sustained maintenance of blood flow to the brain, adrenal gland and the heart at the expense of other tissues. This result in activation of peripheral chemoreceptors, with an increase in sympathetic tone, and in later gestation there is also an increase in concentrations of circulating cortisol, catecholamines, angiotensin II and vasopressin, with a resultant increase in peripheral resistance. Following severe hypoxia there is a shift to anaerobic pathways of metabolism as the “deprived” tissues may not be able to maintain oxidative phosphorylation. [35] Since the fetus has a lesser ability to cope with acid-base disturbances, lactic acidemia can rapidly develop.[35,36] This shifts the oxyhemoglobin dissociation curve to the right, with a further reduction in blood O2 content. Thus, the resulting vicious circle can quickly result in fetal death. Less severe, but chronic hypoxemia is associated with increased erythropoietin concentrations, a slowing of fetal growth, and reduced glycogen stores because of their utilization. Such children have a higher than usual incidence of neurological deficits.[6,26,37-39] The fetal oxyhemoglobin dissociation curve is left-shifted in comparison with the mother’s [Figure 1] because fetal hemoglobin has a lower binding affinity for 2,3-diphosphoglycerate.[2,35] The fetus has a high hemoglobin concentration (16 g/dl), which also contributes to the relatively high oxygen content. The low umbilical PaO2 and fetal vasculature pO2 means that although umbilical venous blood is approximately 85% saturated (compared with approximately 95% in the adult) and has a relatively high oxygen content, the steep dissociation curve allows adequate oxygen unloading to the tissues. The high fetal blood flow allows equivalent or higher oxygen deliveries compared with adults to most tissue beds. However, it appears that fetal PaO2 varies inversely with oxygen consumption, as the capacity to increase oxygen uptake from the lungs is absent.[2,35]
Fetal PaCO2 is high with approximately 60% being carried as bicarbonate. As fetal blood is oxygenated, it releases hydrogen ions, which combine with bicarbonate and push the equilibrium towards CO2 and H2O which diffuse across the placenta, down the concentration gradient for CO2.[2,35] In the maternal circulation, the reaction proceeds in the opposite direction. Maternal pH in the placental blood thus falls slightly while that in the fetal placenta rises slightly. This small rise increases the oxygen affinity of fetal hemoglobin, shifting the oxyhemoglobin dissociation curve further to the left while the maternal curve is shifted right (the “double Bohr shift”). This enhances maternal to fetal oxygen transfer.[2,35]
Fetal and neonatal plasma bicarbonate concentrations are low because of the immaturity of the proximal tubular bicarbonate reabsorption.[25] Buffering via the regulation of bicarbonate is slower than in the adult.
The literature is replete with reports that indicate that asphyxia remains a common cause of intrapartum fetal death.[40] Whilst prematurity and lethal congenital abnormalities accounted for 36% of these and the remaining are due to asphyxia.[40] Many of these deaths might have been avoided by the routine use of continuous fetal heart rate (FHR) monitoring had it been available. However, abnormalities in the FHR pattern are only suggestive of fetal asphyxia, and it has become generally accepted that the pH of a fetal scalp blood sample offers the most reliable indicator of fetal asphyxia in present-day use.[5,6,41-44]
During hypoxia, the fetal pCO2 rises, increasing the level of H2CO3 in the fetal blood and giving rise to a respiratory acidosis and a fall in the blood pH. The combination of hypoxia, hypercapnia and metabolic acidosis constitute asphyxia. At any given level of oxygen depletion, the survival time of the hypoxic fetus will depend primarily on its glycogen reserve. Thus, the well-nourished fetus can be expected to withstand a standard hypoxic insult better than the growth retarded fetus.[14]
The introduction of FBS by Saling (1963),[40,45,46] whereby arterialized capillary blood could be obtained from the fetus during labor, revolutionized the diagnosis of intrapartum fetal asphyxia. Saling (1966) showed that a scalp blood pH <7.20 was associated with poor clinical condition of the infant and he designated values below 7.20 as “acidosis”; he also suggested that a fetal scalp blood pH of between 7.20 and 7.25 were indicative of early fetal deterioration and he designated values within this range as “preacidosis”.[40]
Correlation between the fetal acid-base status and fetal clinical status
Fetal acid-base status in clinical fetal distress and high-risk cases
The classical features of fetal distress which includes meconium staining of the liquor amnii and alteration in the FHR pattern, have been shown not to be sufficiently reliable as an indicator for accurate assessment of the fetus.[12,24] For confirmatory interpretation of the findings, it may have to be combined with fetal blood gas analysis. However, FBS still remains superior to cardiotocography (CTG) alone as it provides direct information about the fetal acid-base balance and gives a more accurate picture of the state of the fetus. Therefore, fetal scalp blood has been found to be suitable for the purpose of predicting the fetal status.[12,24] Good correlation has been established between the fetal pH and the condition of the infant at birth:
i. Fetal distress: It is well evidenced [4,15,24] that in clinical fetal distress, there is a significantly lower fetal pH than that in normal fetuses.
ii. Post-maturity: Cases of post-maturity also gave a lower fetal pH than normal.[24]
iii. Uterine inertia: In labors associated with uterine inertia, it has been consistently demonstrated that the fetal pH is lower than normal. A lower pH in cases of uterine inertia is not unexpected as progressive retraction of the uterus would jeopardize the placental circulation.[16,24]
It must be noted that of the different signs of clinical fetal distress, meconium is the least important and fetal tachycardia is more significant than others.[24] A cautionary note must be added here that some fetuses have transient abnormal acid-base balance (a check within 30 min was normal), and the FHR trace (obtained immediately after the sampling) showed no ominous changes in these cases. Therefore, an abnormal fetal acid-base balance is not uncommon in a lowrisk population in the labor. However, a critical evaluation of the acid-base balance is necessary since in most cases, the abnormal acid-base balance is transient and of a respiratory, innocuous type without concomitant fetal distress.[44]
Fetal acid-base balance in labor
Intrapartum scalp blood sampling
Decelerations occur in at least 50% of labors where electronic fetal monitoring (EFM) is used.[46] Even with the worst pattern of tachycardia, reduced variability and decelerations, only 50-60% of fetuses are acidotic. Acidosis is a function of time with ongoing insult. CTG has high sensitivity but low specificity. Meta-analysis of three trials comparing Intermittent Auscultation with EFM used without FBS showed a fourfold increase in caesarean section rate. This increase was limited to twofold where FBS was used in conjunction with CTG, with no improvement in outcome measures in either group Table 3.
| Clinical techniques have been used to separate pregnant patients into normal and high-risk groups |
| Pregnancy is associated with substantial reduction in the partial pressure of CO2 |
| This change favors diffusion between maternal and fetal circulations |
| Adequate buffering prevents significant changes in maternal arterial pH |
| Very small changes in pH may significantly affect function of various fetal organ systems, such as the central nervous system and the cardiovascular system |
| Fetal hypoxia can occur when maternal oxygenation is compromised, maternal perfusion of the placenta is reduced, or delivery of oxygenated blood from the placenta to the fetus is impeded |
| In presence of hypoxia, complete oxidative metabolism of carbohydrates to CO2 and water is impaired and metabolism proceeds along an anaerobic pathway with production of organic acids, such as lactic acid, which are not readily excreted or metabolized |
| During labor of all patients, a gradual fall in fetal pH and an increase in pCO2 and base deficit were noted, especially in the second stage of labor and during delivery. This acidosis was more marked in high-risk fetuses |
| The high-risk fetus does not tolerate the stresses of labor as well as the normal |
| At birth and during the 1st h of life the neonates born to high-risk mothers were more acidotic and recovered more slowly than neonates born to mothers with normal pregnancies |
| Apgar scores of high-risk babies were statistically significantly lower than babies of normal pregnancies |
| Biophysical and biochemical surveillance of the high-risk fetus may provide early warning of impending difficulties so that timely intervention during labor may be instituted |
| Scalp blood pH is useful to increase specificity of CTG (i.e., to verify the CTG reading) while cord blood pH is useful to differentiate the depressed baby due to hypoxemia/acidosis from other causes |
CTG: Cardiotocography, pCO2: Pressure of carbon dioxide, CO2: Carbon dioxide
Table 3: Main learning points
Decelerations indicate “stress” to the fetus. The addition of a rise in baseline along with reduction in baseline variability indicate a “stress” to “distress” period, but the duration of the “distress” period to the development of metabolic acidosis is unpredictable.[4,23,27,46,47] If FHR develops a “distress” pattern, FBS may be indicated and this may need to be repeated if delivery is not imminent. During the development of subacute hypoxia, a rapid deterioration of pH is possible with no increase in FHR if decelerations involve drop in FHR >60 bpm lasting for 90 s or with less than 60 s of recovery in between. A decline in pH at the rate of 0.01/2-4 min may occur – the decline being more rapid in situations with intrauterine growth restriction, infection, and thick meconium with scanty liquor.[46] It may thus not be appropriate to do FBS if delivery is not likely in a reasonable time.[4]
Alternatives to FBS include scalp stimulation or acoustic stimulation.[46] If accelerations occur in response to stimulation, the fetus is unlikely to have an acidotic pH. In the absence of accelerations in response to stimulation, 50% of fetuses may be acidotic. Other options include fetal electrocardiography ST waveform analysis and fetal pulse oximetry.[46,48]
Umbilical cord blood acid-base assessment
The 26th Royal College of Obstetricians and Gynecologists study group on Intrapartum Fetal Surveillance (1993) [12] recommended measurement of umbilical artery and vein blood pH and base deficit as a measure of fetal condition at birth.[46]
Despite the fact that a wide pH range of 7.00-7.16 is quoted in the literature for acidemia, there is a general consensus that a cord artery pH <7.00 is more significantly correlated with adverse neonatal outcome.[26,34,49,50] The babies with pH <7.00, are more likely to suffer complications in the short term, while long term outcome is correlated to neonatal encephalopathy rather than pH.[46] Also, base deficit is now used as a surrogate to assess metabolic academia-the commonest cut off values to indicate abnormality include BD >8 mmol as moderate and >12 as severe metabolic acidosis.[46]
The principal contributor to metabolic acidosis is lactic acid, which at times of oxygen deficiency is mainly produced by anaerobic glycogenolysis.[12,40] Metabolic acidosis reflects tissue oxygen debt and therefore is of greater prognostic significance than respiratory acidosis. Accumulation of lactic acid together with hypoxia leads to tissue edema and cellular damage.[4,47,51,52] Lactate can be measured in a 5 μl blood sample using an electrochemical strip method with results available by the bedside in 60 s. It is easier to measure than a full blood gas analysis using maintenance free, battery operated pocket size devices like the Lactate Pro (Oxford Sonicaid Ltd.). Lactate is comparable to pH and base deficit with respect to sensitivity, specificity and predictive values of various perinatal complications.[4]
In conclusion, scalp blood pH is useful to increase specificity of CTG (i.e., to verify the CTG reading) while cord blood pH is useful to differentiate the depressed baby due to hypoxemia/ acidosis from other causes.
Correlation between the fetal acid-base status and the Apgar score
With a well-defined setting of a critical pH, the fetal acid-base status correlates well with the Apgar score of the newborn irrespective of the sampling delivery interval. With the criterion of the critical pH set at 7.13, there would be no false results and the error of prediction would be reduced to insignificant values.[5,25,49,50,53]
Challenges in the diagnosis and management of fetal hypoxia (intrapartum asphyxia) in developing countries
Intrauterine fetal hypoxia occurs when the fetus is deprived of an adequate supply of oxygen.[53-55] During hypoxia the fetal pCO2 rises, increasing the level of H2CO3 in the fetal blood and gives rise to a respiratory acidosis and a fall in the blood pH. The combination of hypoxia, hypercapnia and metabolic acidosis constitute asphyxia. At any given level of oxygen depletion, the survival time of the hypoxic fetus will depend primarily on its glycogen reserve. Thus, the well-nourished fetus can be expected to withstand a standard hypoxic insult better than the growth retarded fetus.[14]
Asphyxia remains a common cause of intrapartum fetal death. Whilst prematurity and lethal congenital abnormalities accounted for 36% of these, the remaining are due to asphyxia.[40] The World Health Organization (WHO) estimates that globally, between four and nine million newborns suffer birth asphyxia each year leading to an estimated 1.2 million deaths and about the same number of infants who develop severe disability. WHO estimates for global neonatal deaths caused by birth asphyxia are 29%.[56,57] In Nigeria like most other developing countries, intrauterine hypoxia and birth asphyxia are leading causes of perinatal death.[58-60] And existing data reveals that approximately 4 million fetal deaths occur each year, 98% of them in developing countries.[58-60] These are rough estimates as fetal deaths are infrequently acknowledged or recorded in developing countries.
Intrauterine hypoxia and or birth asphyxia was the ninth most expensive medical condition treated in U.S. hospitals by average hospital cost and resultant hospital charge.[61,62] Though the situation has not been systematically documented in most developing countries like Nigeria; the burden is certainly enormous and probably worse in the developing countries against the backdrop of the poor socioeconomic status of the citizenry.
Clearly from the foregoing, it is imperative that appropriate diagnostic and management modalities for fetal hypoxia be available and accessible. This is the only way the burden of perinatal morbidity and mortality can be reduced with a view to achieving the millennium development goal of improved perinatal outcome. The modalities that can be used for diagnosis include clinical observation of meconium staining of liquor in labor, FHR abnormalities on auscultation or from CTG, as well as FBS for pH assessment and blood gas analysis.[63,64] Very recently, fetal pulse oximetry has gained importance in the intrapartum assessment of the fetus and seems to compare favorably with fetal blood gas analysis.[48] Other assessments are biophysical profile assessment and Doppler studies.[63,64] For the management to be effective, efficient and adequate, skilled birth attendants with proficiency in feto-maternal medicine should be available, and the complimentary neonatal unit to handle the neonates that have suffered fetal hypoxia and subsequent birth asphyxia should also be available. Most of the diagnostic modalities enunciated above are high-tech procedures with the paucity of facilities, and expertise for their use in the few health care facilities where they are available in most developing countries. There is also a paucity of facilities and expertise for neonatal care in most settings in developing nations. Indeed, the public and private primary and secondary health care facilities in most developing nations are devoid of these services. A significant proportion of the tertiary health care facilities also does not have the full complement of diagnostic modalities enumerated above.
Over the immediate past years, a lot of tertiary health facilities have obtained CTG machines for fetal monitoring. This, in addition to the readily available ultrasonography machines has made it possible to undertake biophysical profile studies. Use of FBS for pH and blood gas analysis is still poorly domesticated in these centers; this is inspite of the fact that abnormalities of the FHR pattern are only suggestive of fetal asphyxia, and it has become generally accepted that the pH and blood gas analysis of a fetal scalp blood sample offers the most reliable indicator of fetal asphyxia in present-day use.[5,6,39-43] Also only very few centers undertake Doppler studies due to non-availability of the equipment and expertise.
The readily available diagnostic tools to skilled birth attendants attending to parturients in most developing nations including tertiary health care facilities are clinical observation of meconium staining of liquor and the use of FHR abnormalities from auscultation or CTG. Other modalities such as FBS for pH and blood gas analysis as well as Doppler studies are not readily available. Interestingly, the presence of meconium staining of liquor and or FHR abnormalities on auscultation or CTG monitoring are of high sensitivity, but low specificity,[5] and they have been shown not to be sufficiently reliable for the accurate assessment of the fetus.[12,24] FBS provides direct information about the fetal acid-base balance as well as the blood gas status and gives a more accurate picture of the state of the fetus.[63,64] Fetal scalp blood has been found to be suitable for this purpose.[12,24] Good correlation has been established between the fetal pH and the condition of the infant at birth. It therefore means that the former tests are at best screening tools while the later are diagnostic and confirmatory.
A large proportion of the citizenry in developing nations live in resource poor settings where there are no access to health care facilities, and for those that even live close to the health care facilities, they may not have the economic wherewithal to afford hospital care. Therefore, finance remains yet another major impediment and challenge to accessing requisite diagnostic investigations and the subsequent management of fetal hypoxia in a significant proportion of parturients.
The management of a fetus with established hypoxia includes temporary measures to mitigate the impact of the predisposing conditions if possible such as discontinuing oxytocin infusion in cases of induction or augmentation of labor, displacing the presenting part off the cord in cases of cord prolapse, lying the parturient in the left lateral position and giving oxygen by face mask.[62] The decision to deliver is then made, and this may include a caesarean section and-/or instrumental delivery depending on the stage and progress of the labor. Following delivery, the neonatologist is expected to receive the baby for full evaluation of the newborn and management in the neonatal intensive care unit. Again, a major challenge is the paucity or non-availability of skilled accouchers, neonatologists and neonatal facilities in most public/private primary and secondary health care facilities. This makes it impossible for fetal hypoxia to be picked up early and where they are picked up, the facilities are often not able to appropriately respond to the clinical situation. Finally, in all these the largely poor financial state of the parturients in developing nations may further compound the already bad situation as the policy of health care delivery in most developing countries is based on a “cash and carry” principle.
Conclusion and Summary
Pregnancy is associated with a substantial reduction in the pCO2 and this change favors diffusion between maternal and fetal circulations.[65] Adequate buffering prevents significant changes in maternal arterial pH.
Normal fetal metabolism results in the production of acids which are buffered to maintain extracellular pH within a critical range. Very small changes in pH may significantly affect the function of various organ systems, such as the central nervous system and the cardiovascular system. The major buffers utilized by the fetus for neutralizing hydrogen ion production are plasma bicarbonate and hemoglobin. Inorganic phosphates and erythrocyte bicarbonate are also potential buffers, although they play a lesser role in fetal acid-base hemostasis.
Fetal hypoxia can occur when maternal oxygenation is compromised, maternal perfusion of the placenta is reduced, or delivery of oxygenated blood from the placenta to the fetus is impeded. When adequate fetal oxygenation does not occur, complete oxidative metabolism of carbohydrates to CO2 and water is impaired and metabolism proceeds along an anaerobic pathway with production of organic acids, such as lactic acid, which are not readily excreted or metabolized. Accumulation of lactic acid can deplete the buffer system and result in metabolic acidosis.
The diagnosis and management of fetal hypoxia remains a major challenge in developing nations due to paucity of facilities and skilled personnel coupled with overriding ravaging effect of poverty.
Source of Support: Nil.
Conflict of Interest: None declared.
References
- Dunlop W. Normal pregnancy: Physiology and endocrinology. In: Edmonds DK, editor. Dewhurst’s Textbook of Obstetrics and Gynecology for Postgraduates. 6th ed.: Blackwell Sciences Ltd.;United Kingdom, London. 1999 p. 76-90.
- Blechner JN. Maternal-fetal acid-base physiology. Clin Obstet Gynecol 1993;36:3-12.
- Myers VC, Muntwyler E, Bill AH. The acid-base balance disturbance of pregnancy. J Biol Chem 1931;98:253-60.
- Bobrow CS, Soothill PW. Causes and consequences of fetal acidosis. Arch Dis Child Fetal Neonatal Ed 1999;80:F246-9.
- Lee KH. Correlation between the foetal acid-base status and the Apgar score. Postgrad Med J 1972;48:405-8.
- Gilstrap LC 3rd, Leveno KJ, Burris J, Williams ML, Little BB. Diagnosis of birth asphyxia on the basis of fetal pH, Apgar score, and newborn cerebral dysfunction. Am J Obstet Gynecol 1989;161:825-30.
- Johnson JW, Richards DS. The etiology of fetal acidosis as determined by umbilical cord acid-base studies. Am J Obstet Gynecol 1997;177:274-80.
- Apgar V. A proposal for a new method of evaluation of the newborn infant. Curr Res Anesth Analg 1953;32:260-7.
- Casey BM, McIntire DD, Leveno KJ. The continuing value of the Apgar score for the assessment of newborn infants. N Engl J Med 2001;344:467-71.
- Schifrin BS. The rationale for antepartum fetal heart rate monitoring. J Reprod Med 1979;23:213-21.
- MacRae DJ. Maternal influence on foetal acid-base balance.Proc R Soc Med 1968;61:490-1.
- Recommendations arising from the 26th RCOG study group on Intrapartum Fetal Surveillance. London: Royal College of Obstetricians and Gynaecologists. In: Spencer JAD, ed. Intrapartum fetal surveillance. London: RCOG press; 1993:387-393.
- Utility of umbilical cord blood acid-base assessment. ACOG Committee Opinion: Committee on Obstetric Practice. Number 138 – April 1994. Int J Gynaecol Obstet 1994;45:303-4.
- Goldaber KG, Gilstrap LC 3rd. Correlations between obstetric clinical events and umbilical cord blood acid-base and blood gas values. Clin Obstet Gynecol 1993;36:47-59.
- Low JA. The role of blood gas and acid-base assessment in the diagnosis of intrapartum fetal asphyxia. Am J Obstet Gynecol 1988;159:1235-40.
- Hagelin A, Leyon J. The effect of labor on the acid-base status of the newborn. Acta Obstet Gynecol Scand 1998;77:841-4.
- Huckabee WE, Metcalfe J, Prystowsky H, Barron DH.Insufficiency of O2 supply to pregnant uterus. Am J Physiol 1962;202:198-204.
- Blechner JN, Stenger VG, Eitzman DV, Prystowsky H. Effects of maternal metabolic acidosis on the human fetus and newborn infant. Am J Obstet Gynecol 1967;99:46-54.
- Motoyama EK, Rivard G, Acheson F, Cook CD. Adverse effect of maternal hyperventilation on the foetus. Lancet 1966;1:286-8.
- Moya F, Morishima HO, Shnider SM, James LS. Influence of maternal hyperventilation on the newborn infant. Am J Obstet Gynecol 1965;91:76-84.
- Rivard G, Motoyama EK, Acheson FM, Cook CD, Reynords EO. Effects of maternal hyperventilation on the experimental animal. Am J Obstet Gynecol 1967;97:925.
- Panigel M. Placental perfusion experiments. Obstet Gynecol 1962;84:1604.
- Modanlou H, Yeh SY, Hon EH. Fetal and neonatal acid-base balance in normal and high-risk pregnancies: During labor and the first hour of life. Obstet Gynecol 1974;43:347-53.
- Lee KH. Foetal acid-base status in clinical foetal distress and high risk cases. Postgrad Med J 1972;48:91-4.
- Low JA, Lindsay BG, Derrick EJ. Threshold of metabolic acidosis associated with newborn complications. Am J Obstet Gynecol 1997;177:1391-4.
- Victory R, Penava D, Da Silva O, Natale R, Richardson B. Umbilical cord pH and base excess values in relation to adverse outcome events for infants delivering at term. Am J Obstet Gynecol 2004;191:2021-8.
- Fahey J, King TL. Intrauterine asphyxia: Clinical implications for providers of intrapartum care. J Midwifery Womens Health 2005;50:498-506.
- Clapp JF 3rd. The relationship between blood flow and oxygen uptake in the uterine and umbilical circulations. Am J Obstet Gynecol 1978;132:410-3.
- Itskovitz J, LaGamma EF, Rudolph AM. The effect of reducing umbilical blood flow on fetal oxygenation. Am J Obstet Gynecol 1983;145:813-8.
- Soothill PW, Nicolaides KH, Campbell S. Prenatal asphyxia, hyperlacticaemia, hypoglycaemia, and erythroblastosis in growth retarded fetuses. Br Med J (Clin Res Ed) 1987;294:1051-3.
- Khong TY, De Wolf F, Robertson WB, Brosens I. Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. Br J Obstet Gynaecol 1986;93:1049-59.
- Wilkening RB, Meschia G. Fetal oxygen uptake, oxygenation, and acid-base balance as a function of uterine blood flow. AmJ Physiol 1983;244:H749-55.
- Soothill PW, Nicolaides KH, Rodeck CH. Effect of anaemia on fetal acid-base status. Br J Obstet Gynaecol 1987;94:880-3.
- Vandenbussche FP, Van Kamp IL, Oepkes D, Hermans J, Bennebroek Gravenhorst J, Kanhai HH. Blood gas and pH in the human fetus with severe anemia. Fetal Diagn Ther 1998;13:115-22.
- Pipkin FB. Fetal growth and physiology. In: Edmonds KD, editor. Dewhurst’s Textbook of Obstetrics and Gynecology for Postgraduates. 6th ed. Blackwell Science Ltd.;United Kingdom. 1999. p. 104-12.
- Goldaber KG, Gilstrap LC 3rd, Leveno KJ, Dax JS, McIntire DD. Pathologic fetal acidemia. Obstet Gynecol 1991;78:1103-7.
- Low JA, Panagiotopoulos C, Derrick EJ. Newborn complications after intrapartum asphyxia with metabolic acidosis in the term fetus. Am J Obstet Gynecol 1994;170:1081-7.
- Ruth VJ, Raivio KO. Perinatal brain damage: Predictive value of metabolic acidosis and the Apgar score. BMJ 1988;297:24-7.
- Fee SC, Malee K, Deddish R, Minogue JP, Socol ML. Severe acidosis and subsequent neurologic status. Am J Obstet Gynecol 1990;162:802-6.
- Pearson JF. Fetal blood sampling and gas exchange. J Clin Pathol 1976;29 Suppl 10:31-4.
- Vintzileos AM, Knuppel RA. Multiple parameter biophysical testing in the prediction of fetal acid-base status. Clin Perinatol 1994;21:823-48.
- Wood C, Ferguson R, Leeton J, Newman W, Walker A. Fetal heart rate and acid-base status in the assessment of fetal hypoxia. Am J Obstet Gynecol 1967;98:62-70.
- Ecker JL, Parer JT. Obstetric evaluation of fetal acid-base balance. Crit Rev Clin Lab Sci 1999;36:407-51.
- Ingemarsson I, Arulkumaran S. Fetal acid-base balance in low-risk patients in labor. Am J Obstet Gynecol 1986;155:66-9.
- Saling, E. (1963). Die Blutgasverhiiltnisse und der SaureBasen-Haushalt des Feten bei ungestortem Geburtsablauf. Z. Geburtsh. Gyndk., 161, 262-292.
- Saling, E. (1966). Aminoscopy and foetal blood sampling. Observations on foetal acidosis. Arch. Dis. Childh., 41, 472-476.
- Tamizian O, Arulkumaran S. Fetal acid-balance in labor. Academic Department of Obstetrics and Gynecology, Derby City General Hospital, Uttoxeter Road, Derby DE22 3NE.
- Sehdev HM, Stamilio DM, Macones GA, Graham E, Morgan MA. Predictive factors for neonatal morbidity in neonates with an umbilical arterial cord pH less than 7.00. Am J Obstet Gynecol 1997;177:1030-4.
- Bakr AF, Al-Abd M, Karkour T. Fetal pulse oximetry and neonatal outcome: A study in a developing country. J Perinatol 2005;25:759-62.
- van den Berg PP, Nelen WL, Jongsma HW, Nijland R, Kollée LA, Nijhuis JG, et al. Neonatal complications in newborns with an umbilical artery pH <7.00. Am J Obstet Gynecol 1996;175:1152-7.
- Landon MB. Acid-base disorders during pregnancy. Clin Obstet Gynecol 1994;37:16-24.
- Huch R, Huch A. Maternal-fetal acid-base balance and blood gas measurement. In: Beard RW, Nathanielsz PW, editors. Fetal Physiology and Medicine. New York: Marcel Dekker; 1984. p. 713.
- Goodwin TM, Belai I, Hernandez P, Durand M, Paul RH. Asphyxial complications in the term newborn with severe umbilical acidemia. Am J Obstet Gynecol 1992;167:1506-12.
- Blumenthal I. Cerebral palsy – Medicolegal aspects. J R Soc Med 2001;94:624-7.
- Dhar KK, Ray SN, Dhall GI. Significance of nuchal cord. JIndian Med Assoc 1995;93:451-3.
- Committee on Obstetric Practice, American College of Obstetricians and Gynecologists. ACOG Committee Opinion. Number 326, December 2005. Inappropriate use of the terms fetal distress and birth asphyxia. Obstet Gynecol 2005;106:1469-70.
- Hamilton BE, Martin JA, Ventura SJ. Births: Preliminary data for 2012. National vital statistics reports; Vol 62 no 3. Hyattsville, MD: National Center for Health Statistics, 2013.
- Bang AT, Bang RA, Baitule SB, Reddy HM, Deshmukh MD. Management of birth asphyxia in home deliveries in rural Gadchiroli: The effect of two types of birth attendants and of resuscitating with mouth-to-mouth, tube-mask or bag-mask. J Perinatol 2005;25 Suppl 1:S82-91.
- Kuti O, Orji EO, Ogunlola IO. Analysis of perinatal mortality in a Nigerian teaching hospital. J Obstet Gynaecol 2003;23:512-4.
- Ezechukwu CE, Ugochukwu EF, Egbuonu I, Chukwuka JO. Risk factor for neonatal mortality in a regional tertiary hospital in Nigeria. Niger J Clin Pract 2004;7:50-2.
- The Nigerian Academy of Science. In: Nwosu J, Odubanjo MO, Osinusi BO, editors. Reducing Maternal and Infant Mortality in Nigeria (Workshop Summary). Lagos, Nigeria: West African Book Publishers; 2009.
- Rueda-Clausen CF, Morton JS, Davidge ST. Effects of hypoxia- induced intrauterine growth restriction on cardiopulmonary structure and function during adulthood. Cardiovasc Res 2009;81:713-22.
- Sly PD, Drew JH. Massive pulmonary haemorrhage: A cause of sudden unexpected deaths in severely growth retarded infants. Aust Paediatr J 1981;17:32-4.
- Low JA. Fetal monitoring in labor. In: Edmond DK, editor. Dewhurst Textbook of Obstetrics and Gynaecology for postgraduates. 7th ed. Blackwell Sciences Ltd.; United Kingdom, 2007. p. 56-62.
- Spencer JA. Intrapartum fetal monitoring. In: Edmond DK, editor. Dewhurst Textbook of Obstetrics and Gynaecology for Postgraduates. 6th ed. Blackwell Sciences Ltd.; United Kingdom, 1999. p. 259-76.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.