Musculoskeletal Complications Among Children with Sickle Cell Anemia Admitted in University of Nigeria Teaching Hospital Ituku ‑ Ozalla Enugu: A 58 Month Review
- *Corresponding Author:
- Dr. Josephat Chinawa Maduabuchi
Department of Pediatrics, College of Medicine, University of Nigeria Enugu Campus, Enugu, Nigeria.
E-mail: josephat.chinawa@unn.edu.ng
Citation: Chinawa JM, Chukwu BF, Ikefuna AN, Emodi IJ. Musculoskeletal complications among children with sickle cell anemia admitted in university of Nigeria Teaching Hospital Ituku - Ozalla Enugu: A 58 month review. Ann Med Health Sci Res 2013;3:564-7.
Abstract
Background: Sickle cell anemia (SCA) is a genetic hematological disorder characterized by red blood cells that assume abnormal, rigid, and sickle shape. The musculoskeletal complications of SCA result from vessel occlusion, leading to tissue ischemia and infarction and progressive end organ damage. Aim: The aim of this study was to determine the pattern of musculoskeletal complications among children with SCA admitted at the University of Nigeria Teaching Hospital (UNTH) Ituku Ozalla. Subjects and Methods: This is a retrospective study of cases of musculoskeletal complications among SCA patients admitted in UNTH over a 58 month period. Data were analyzed using the SPSS version 20 and GraphPad Prism 5 soft‑wares. Variables analyzed include musculoskeletal complications, age and gender distribution of subjects. Fisher’s exact was used to test for significant association of categorical variables while Mann Whitney U‑test was used to compare the means. A P value of less than 0.05 was accepted as significant. Results: There were 300 registered patients in our sickle cell clinic. A total of 78 patients (52 males and 26 females) were admitted between January 2007 and October 2012. The median age of the patients was 10 years (range: 9 months to 17 years). 25 of the 78 patients had musculoskeletal complications, giving a prevalence rate of 32.1%. The most common musculoskeletal complication was acute osteomyelitis, diagnosed in 10/78 (12.8%) of the patients. Conclusion: Acute osteomyelitis is the most common musculoskeletal complications in children with SCA attending UNTH Ituku Ozalla.
Keywords
Children, Musculoskeletal complications, Sickle cell anemia
Background
Sickle cell anemia (SCA) is an autosomal recessive genetic blood disorder characterized by red blood cells that assume an abnormal, rigid, and sickle shape. Sickling decreases the cells’ flexibility and results in a risk of various complications.[1]
Sickle cell disorders were originally found in the tropics and subtropics but are now common world‑wide due to migration of people from tropical to the temperate zone.[2] The prevalence of SCA in Nigeria ranges from 0.4% to 3%, affecting about 20 per thousand new‑borns.[3] About 85% of sickle cell disorders and over 70% of all affected births occur in Africa.[4] It is noted that at least 5.2% of the world population have sickle cell trait.[4] Musculoskeletal complications of SCA result from vessel occlusion, leading to tissue ischemia, infarction, progressive end organ damage, and bone marrow hyperplasia.[5] Bone marrow hyperplasia results in widening of the medulla and subsequent cortical thinning. This results in coarsening of the normal trabecular pattern, with loss of cortico‑medullary differentiation in both long and flat bones.[5‑8] Musculoskeletal complications are often observed in the evolution of the disease. These include osteomyelitis, aseptic necrosis of the femoral head, septic arthritis, soft‑tissues abnormalities (such as leg ulcers, myonecrosis, pyomyositis, dactylits, and growth abnormalities) pathological fractures and growth defects.[9‑11]
This study therefore aims at determining the pattern of musculoskeletal complications in children with SCA admitted at the University of Nigeria Teaching Hospital (UNTH), Ituku Ozalla Enugu. The findings from this study will add to the increasing knowledge and management of this challenging disease.
Subjects and Methods
Study area
This study was carried out at the UNTH, Ituku‑Ozalla Enugu. It is a referral center for various health centers in Enugu state and her environs. Enugu state is in the South‑East geopolitical zone of Nigeria.
Study population
There are about 300 children registered at the sickle cell clinic of UNTH Enugu.
Diagnostic criteria
Diagnostic criteria of avascular necrosis of head of femur in this study involve painful limitation of hip motion with restriction of passive movements of the hip. This was then confirmed by antero‑posterior radiographs of the affected hip.[12]
Chronic osteomyelitis (COM), on the other hand was diagnosed as a relapsing and persistent infection that evolves over months to years characterized by low‑grade inflammation, presence of dead bone (sequestrum), new bone apposition, and fistulous tracts.[13,14] Diagnosis of wedge collapse of the vertebra with Gibbus and fractured left 5th metacarpal bone were made radiographically.
Ethical consideration and consent
Ethical clearance for the study was sought from the Health Research and Ethics Committee of the UNTH Ituku Ozalla.
Case selection
This was a retrospective study. SCA patients with musculoskeletal complications admitted in our hospital from January 2007 to December 2012 were included in the study. Their medical history were retrieved from the hospital’s medical records department and examined individually by the investigators. Subjects included in this study were patients aged 6 months to 18 years with hemoglobin genotype SS (diagnosed by cellulose acetate electrophoresis at pH 8.6). Children with SCA who had any congenital musculoskeletal abnormalities or incomplete data or unclear diagnosis were excluded.
The objective of this study was to determine the pattern, age and gender distribution of musculoskeletal complications of SCA in these children.
Data analysis
Data were analyzed by using the SPSS version 20 (Chicago IL, USA) and Graphpad Prism 5 soft‑wares. An initial frequency count of all variables was carried out. Variables analyzed included the various musculoskeletal complications; their age and gender distributions. Fisher’s exact was used to test for significant association of categorical variables while Mann Whitney U‑test was used to compare the means of continuous variables. Data presentation was in the form of tables.
All reported P values were 2‑sided and values less than 0.05 were accepted as level of significant.
Results
A total of 300 patients were registered in our sickle cell clinic. A total of 78 patients were admitted from January 2007 to October 2012. Fifty‑two of the 78 patients (66.7%) were males and 26/78 (33.3%) were females. Median age of the patients was 10 years (9 months to 17 years). Table 1 depicts the demographic profile of the patients.
| Age | Gender | Total (%) | |
|---|---|---|---|
| Male (%) | Female (%) | ||
| <1 | 1(1.3) | 0 (0) | 1(1.3) |
| 1‑5 | 12(15.4) | 7(8.9) | 19(24.4) |
| 6‑10 | 12(15.4) | 11(14.1) | 23(29.5) |
| 11‑15 | 18(23.1) | 8 (10.2) | 26(33.3) |
| >15 | 9 (11.5) | 0 (0) | 9(11.5) |
| Total | 52(66.7) | 26(33.2) | 78(100.0) |
Table 1: Age and gender distribution of studied patients
Twenty five of the 78 (32.1%) patients had musculoskeletal complications. Median age of patients with musculoskeletal complications was 10 years while that of those without complications was 9 years, which showed no significant difference (Mann‑Whitney U‑test, P = 0.24). Common musculoskeletal symptoms included lower limb pain seen in 39 (50.0%) of cases and upper limb pain in 36 (46.2%).
The most common musculoskeletal complication was acute osteomyelitis, diagnosed in 10/78 (12.8%) of the patients. COM and avascular necrosis of head of femur were each diagnosed in 5/78 (6.4%) patients. Septic arthritis was diagnosed in 2/78 (2.6%) patients while 1/78 (1.3%) had each of chronic leg ulcer, pathological fracture, and vertebral collapse.
None of the complications occurred in infancy, 6 occurred in under‑fives, 7 in childhood and the remaining 12 occurred in adolescence. There was no significant difference in the age group of patients with and without complications (P = 0.99) as well as in the median age of patients with specific musculoskeletal complications (Kruskal‑Wallis test, P = 0.14. The median age of patients with acute osteomyelitis was 9 years while that of those with COM was 5 years, with no significant difference (Mann‑Whitntey U‑test, P = 0.27). The median age of patients with avascular necrosis was 14 years and 9.5 years for septic arthritis. 5 cases of avascular necrosis occurred at the femoral head (4 had X‑ray support) and all the affected patients were 10 years and above. Chronic leg ulcer and fracture of the base of left 5th metacarpal bone were each seen in the 16 year olds. Table 2 shows the age distribution of the specific musculoskeletal complications.
| Complication | Age in years | Total | ||||
|---|---|---|---|---|---|---|
| <1 | 1‑5 | 6‑10 | 11‑15 | >15 | ||
| Acute osteomyelitis | 0 | 3 | 3 | 4 | 0 | 10 |
| Chronic osteomyelitis | 0 | 3 | 1 | 1 | 0 | 5 |
| Avascular necrosis | 0 | 0 | 1 | 3 | 1 | 5 |
| Septic arthritis | 0 | 0 | 1 | 1 | 0 | 2 |
| Chronic leg ulcer | 0 | 0 | 0 | 0 | 1 | 1 |
| Pathological fracture | 0 | 0 | 0 | 0 | 1 | 1 |
| Vertebral collapse | 0 | 0 | 1 | 0 | 0 | 1 |
| Total | 0 | 6 | 7 | 9 | 3 | 25 |
Table 2: Musculoskeletal complications among age groups
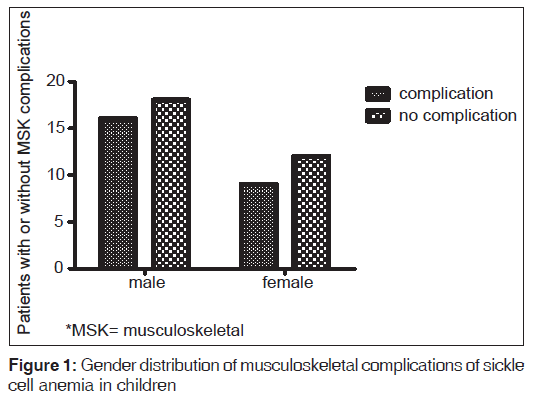
Gender did not have any significant effect on the occurrence of these complications. Sixteen of 52 (30.7%) males developed complications compared to 34.6% (9/26) of females (Fischer’s exact test, P = 0.66). Out of the ten cases of acute osteomyelitis, 6 (60.0%) occurred in males while 4 (40%) were in females. Five cases of avascular necrosis were seen, 2 (40.0%) and 3 (60.0%) occurred in males and females respectively‑ Table 3 and Figure 1.
| Complication | Male | Female | Total |
|---|---|---|---|
| Acute osteomyelitis | 6 | 4 | 10 |
| Chronic osteomyelitis | 3 | 2 | 5 |
| Avascular necrosis | 2 | 3 | 5 |
| Septic arthritis | 2 | 0 | 2 |
| Chronic leg ulcer | 1 | 0 | 1 |
| Pathological fracture | 1 | 0 | 1 |
| Vertebral collapse | 1 | 0 | 1 |
| Total | 16 | 9 | 25 |
Table 3: Musculoskeletal complications among gender groups
One case of COM had positive culture of Staphylococcus aureus from the discharging sinus. Only patients with avascular necrosis of the femoral head had residual disease while the rest had complete resolution of the complications after treatment.
Discussion
The prevalence of musculoskeletal complications in our SCA patients is 32.1%.This prevalence varied with that of Dipty15 in India and Bahebeck[16] in Yaounde Bahebeck[15] in Dipty et al.[16] in India who reported prevalences of 7.8% and 41.6% respectively. The reason for this variation could be geographical and sample size difference. The most common musculoskeletal complication was acute osteomyelitis with prevalence of 12.8%. This prevalence varied with that of Ejindu et al.[5] who reported prevalence of 18% and Bahebeck et al.[15] in Yaounde. The increased susceptibility of sickle cell disease patients to osteomyelitis, has long been recognized with several mechanisms postulated including hyposplenism, impaired complement activity, and the presence of infarcted or necrotic bone.[5,17,18]
We cultured S. aerus in blood samples of children with acute SCA who had acute osteomylitis. This varied slightly from what is commonly obtained. Osteomyelitis in sickle cell disease has also been reported in association with tuberculosis due systemic spread of Mycobacterium ulcerans from a buruli skin ulcer.[19‑22] None of our patients had tuberculosis or buruli ulcer
We noted that the majority of our patients had radiographs, which aided in the diagnosis of musculoskeletal complications. Radiographs are very important in diagnosing musculoskeletal disorders in SCA, for instance some of our patient who presented with diffuse lower limb pains that mimicked vaso‑occlusive episode surprisingly showed osteomyelitis in radiographs. This is buttressed by the work of Ware et al.[10] who noted that in avascular necroses of bones, the initial radiographs could be normal, but thereafter typical appearance of sclerosis, subchondrial collapse and flattening were seen. In addition, clinical and radiographic features of acute osteomyelitis may be particularly difficult to distinguish from those of bone infarction. In that context, magnetic resonance (MR) imaging may be useful. At MR imaging, findings of cortical defects, adjacent fluid collections in soft‑tissue, and bone marrow enhancement are suggestive of infection.[15] MR imagery was not performed in our series due to lack of facility.
We noted from this work that musculoskeletal complication become more pronounced as children with SCA increases in age. This could be due to worsening hypoxemia, infarction, and maturation of the coagulation system from infancy to adulthood (termed developmental homeostasis).[22]
Conclusion
The prevalence of musculoskeletal complications in children with SCA attending UNTH Ituku Ozalla was 32.1%.Acute osteomyelitis was the most common complications. Musculoskeletal complications tend to manifest as patient advances in age.
Strength and limitation
A major strength of our study is the long study duration (58 months) that focused solely on children with SCA. We were however, unable to do investigation such as radionuclide and MR imaging’s due to lack of facilities. These would have helped the sensitivities of our diagnostic criteria. The small sample size and inability to explore other risk factors also limited this work and its generalizability.
Acknowledgements
Our gratitude extends to all the staff of the Health Records Department of the UNTH and nurses for their support and co-operation.
References
- Quirolo K, Vichinsky E. Haemoglobin disorders. In: Behrmen RE, Kliegman EM, Jenson HB, editors. Nelson Text Book of Paediatrics. 17th ed. Philadephia: Saunders Company; 2004. p. 1623‑34.
- Ahmed SG, Bukar AA, Jolayemi B. Hematological indices of sickle cell anaemia patients with pulmonary tuberculosis in northern Nigeria. Mediterr J Hematol Infect Dis 2010;2:e2010014.
- Weatherall DJ, Clegg JB. Inherited haemoglobin disorders: An increasing global health problem. Bull World Health Organ 2001;79:704‑12.
- Modell B, Darlison M. Global epidemiology of haemoglobin disorders and derived service indicators. Bull World Health Organ 2008;86:480‑7.
- Ejindu VC, Hine AL, Mashayekhi M, Shorvon PJ, Misra RR. Musculoskeletal manifestations of sickle cell disease. Radiographics 2007;27:1005‑21.
- Soyanwo OA, Bamgbade YA. Anaesthesia and sickle cell disease. Nig Quart J Hosp Med 1999;9:21‑3.
- Conran N, Franco‑Penteado CF, Costa FF. Newer aspects of the pathophysiology of sickle cell disease vaso‑occlusion. Hemoglobin 2009;33:1‑16.
- Kim SK, Miller JH. Natural history and distribution of bone and bone marrow infarction in sickle hemoglobinopathies. J Nucl Med 2002;43:896‑900.
- Snakaran M, Sandat AM, Kannen K. Septic arthritis in sickle cell disease. Int Orthop 1988;12:225‑57.
- Ware HE, Brooks AP, Toye R, Berney SI. Sickle cell disease and silent avascular necrosis of the hip. J Bone Joint Surg Br 1991;73:947‑9.
- Almeida A, Roberts I. Bone involvement in sickle cell disease. Br J Haematol 2005;129:482‑90.
- Mont MA, Jones LC, Hungerford DS. Nontraumatic osteonecrosis of the femoral head: Ten years later. J Bone Joint Surg Am 2006;88:1117‑32.
- Zuluaga AF, Galvis W, Saldarriaga JG, Agudelo M, Salazar BE, Vesga O. Etiologic diagnosis of chronic osteomyelitis: A prospective study. Arch Intern Med 2006;166:95‑100.
- Ballas SK, Lieff S, Benjamin LJ, Dampier CD, Heeney MM, Hoppe C, et al. Definitions of the phenotypic manifestations of sickle cell disease. Am J Hematol 2010;85:6‑13.
- Dipty J, Khushnooma I, Vijaya S, Kanjaksha G, Roshan C. Sickle cell anemia from central India: A Retrospective analysis. Indian Pediatr 2012;49:911-3.
- Bahebeck J, Atangana R, Techa A, Monny‑Lobe M, Sosso M, Hoffmeyer P. Relative rates and features of musculoskeletal complications in adult sicklers. Acta Orthop Belg 2004;70:107‑11.
- Platt OS, Thorington BD, Brambilla DJ, Milner PF, Rosse WF, Vichinsky E, et al. Pain in sickle cell disease. Rates and risk factors. N Engl J Med 1991;325:11‑6.
- Chambers JB, Forsythe DA, Bertrand SL, Iwinski HJ, Steflik DE. Retrospective review of osteoarticular infections in a pediatric sickle cell age group. J Pediatr Orthop 2000;20:682‑5.
- Pszolla N, Sarkar MR, Strecker W, Kern P, Kinzl L, Meyers WM, et al. Buruli ulcer: A systemic disease. Clin Infect Dis 2003;37:e78‑82.
- Wang WC. Sickle cell anaemia and other sickling syndromes. In: Green JP, Forester J, Lukens JN, editors. Wintrobes Clinical Haematology. 4th ed. Philadephia: Lipinkott, William and Wilkson; 2004. p. 1263‑311.
- Omojola MF, Annobil S, Adzaku F, Addae SK, Mohammed S. Bone changes in sickle cell anaemia. East Afr Med J 1993;70:154‑8.
- Monagle P, Barnes C, Ignjatovic V, Furmedge J, Newall F, Chan A, et al. Developmental haemostasis. Impact for clinical haemostasis laboratories. Thromb Haemost 2006;95:362‑72.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.