Neonatal Malaria in the Gambia
- *Corresponding Author:
- Dr Herbert A Obu
P O Box 14587 Enugu, Nigeria.
E-mail: herbert.obu@unn.edu.ng
Date of Received :12/10/2010
Date of revised :26/12/2010
Date of Accepted :28/12/2010
Abstract
Background: Neonatal malaria was thought to be uncommon, even in malaria endemic areas. Neonates with febrile illness and / or related symptoms are often presumed to have neonatal septicaemia and examination of blood films for malaria parasites is rarely included in the initial work-up of these babies.
Objective: To determine the existence, or otherwise, and the clinical features of malaria in neonates with clinical features suggestive of septicaemia.
Patients and Methods: In a prospective study, all consecutive babies admitted in the neonatal unit of the Royal Victoria Hospital over an eighth month period with an initial diagnosis of neonatal septicaemia were screened for malaria in addition to an extensive sepsis work-up. Malaria parasitaemia was diagnosed on Giemsa stained blood smears.
Results: One hundred and five babies admitted in the neonatal unit of the Royal Victoria Hospital, The Gambia with an initial diagnosis of neonatal septicaemia were studied. Fourteen (13.3%) of these babies had positive blood smears for malaria parasites and bacterial cultures of their blood, cerebrospinal fluid and urine yielded no pathogen. Plasmodium falciparum was the only species identified in all the babies. A male preponderance was obtained with a male: female ratio of 2.5:1. Malaria occurred more commonly in babies aged more than one week, thus suggesting that acquired rather than congenital malaria was the more common form of neonatal malaria in the area. All the babies with malaria were admitted within the rainy season period. The principal clinical features observed in these babies were fever, refusal to feed, maternal fever in the last trimester, excessive crying, difficulty in breathing, vomiting, hepatomegaly, abdominal distension and irritability. A similar picture was observed among babies with proven neonatal septicaemia. In addition, depressed primitive reflexes was found to be more common in babies with septicaemia (p=0.01).
Conclusions: These data show that neonatal malaria is not uncommon in The Gambia and that the clinical features are akin to those of neonatal septicaemia.
Key words
Malaria; septicaemia; neonate: The Gambia.
Introduction
Malaria, also known as “ague” in the olden days, has been described since ancient times. Hippocrates, the father of modern medicine, is usually credited with the first clear description among occidental writers.[1] In his aphorisms, he described the regular paroxysms of fever now known to be associated with clinical malaria. In Europe seasonal periodic fevers were particularly common in marshy areas and Italian writers had thought that malaria was caused by the offensive vapours emanating from the Tiberian marshes, hence the terms "paludism" (L. palus = marsh) and "mal aria" (bad air).[2]
The disease is caused by the invasion of red blood cells by protozoan parasites of the genus Plasmodium. Almost all deaths and severe disease due to malaria are caused by P. falciparum.[3]
More than 200 million people are affected by malaria worldwide. It is endemic in over ninety countries and is responsible for between one and three million deaths per year. Children, especially African children, carry the greatest burden of the disease with an estimated two hundred million episodes of clinical malaria each year.[3-7] The vast majority of malaria associated deaths occur in this category of people.[6] In The Gambia, malaria is responsible for approximately twenty-five per cent of deaths that occur in children below five years of age.[7, 8]
It used to be believed that neonates in malaria endemic areas were protected from attacks of malaria. This protection was attributed to various mechanisms such as the milk diet of the infant being deficient in p-amino-benzoic acid, [9] haematological factors such as an aging red cell population, the presence of erythrocyte foetal haemoglobin, [10] and selective biting by mosquitoes among different age-groups.[11] Transplacentally acquired (maternal) antibodies have also been suggested to play a major role in the protection of the newborn.[11-12]
More recent reports are beginning to show that this protection may not be complete and that symptomatic malaria in the newborn period can occur, and that such babies may present with severe disease.[13-23]
In 1970 Logie and McGregor reported that in more than twenty years of their experience in The Gambia no case of malaria in the newborn was seen.[24] Time has passed since 1970 and the situation may have changed.
This study thus sought to determine the existence and the features of neonatal malaria as seen at the Royal Victoria Hospital in Banjul, The Gambia.
Materials and Methods
This prospective study was conducted at the Paediatric Unit of the Royal Victoria Hospital, Banjul, The Gambia over a period of eight months, June 1998 to January 1999 inclusive, covering parts of the rainy and dry seasons. The minimum sample size for the study was calculated from the formula:
n = (z1-a)2 x (p) x (1-p)/d2, 25
where n = minimum sample size
p = best estimate of the population prevalence obtained from the literature review (23.7%) [26]
d = the difference between the true population rate and the sample rate that can be tolerated = 1%
z1-a = 1.96 (at 95% confidence level).
The minimum sample size thus calculated was 70. However, to make room for an assumed attrition rate of 50%, [25] an additional number of 35 patients were recruited thus bringing the total number of patients studied to 105.
Inclusion Criteria
All consecutive neonates (age 0 - 28 days) admitted into the New Born Special Care Unit (NBSCU) of the hospital with features suggestive of neonatal septicaemia were included in the study.
Exclusion Criteria
1. Age > 28 days.
2. Neonates admitted into the unit for reasons other than neonatal septicaemia.
3. Patients admitted for other conditions (e.g. asphyxia, etc.) but who developed features of septicaemia while on admission.
4. Patients belonging to categories 2 and 3 above were excluded in order to avoid, or at least minimise, the effects of confounding factors.
5. Patients who had received more than 2 doses of chloroquine, quinine or other anti malarial drugs within 48 hours before admission.
All consecutive neonates (age 0 - 28 days) admitted in the neonatal unit of the hospital with features suggestive of neonatal septicaemia were enrolled in the study until the desired sample size was obtained.
Detailed clinical history, including history of antenatal and perinatal events, was obtained for every baby enrolled in the study. Thorough physical examination, including anthropometric measurements, was also done for each of the babies.
The gestational age of the babies was determined by a combination of the maternal dates (when available) and gestational age assessment using the methods of Dubowitz.27 (whenappropriate).
The laboratory studies done for all the patients enrolled in the study included complete blood counts, blood cultures (aerobic and anaerobic), Cerebrospinal Fluid (CSF) studies (cultures / other analysis), urine analysis, microscopy and cultures (urine specimens collected via suprapubic punctures), umbilical swab cultures, wound swabs (when indicated) as well as examination of thick and thin blood smears for malaria parasites. Examination for malaria parasites was done consecutively for the first three days of admission in all the patients. Thick and thin blood smears were prepared and stained with Giemsa [28] and microscopic examinations of the blood smears were done at x100 oil immersion magnification. The absence of malaria parasites was not reported until at least two hundred fields of a thick film had been examined.[28] The results were taken as positive if any of the three consecutive samples revealed malaria parasites and as negative if none of the three consecutive samples revealed malaria parasites. The density of parasitaemia in the thick blood films was reported as follows:
+ 1 parasite/field
++ 2-19 parasites/field
+++ > 20 parasites/field.[28]
All the patients studied were empirically commenced on treatment with intra venous ampicillin and gentamicin, except when malarial parasitaemia was detected on the first day of admission and only antimalarial treatment was given. Patients with malaria parasitaemia and in whom all cultures were negative for bacterial agents were treated with oral chloroquine 5 mg/kg body weight per day for 5 days while antibiotics were withdrawn as soon as the results are obtained.
Results were collected with the aid of forms designed for the study. Data management was done with the aid of Epi-Info version 6 statistical software, and the level of significance was set at 95% confidence level.
Ethical consent for the study was obtained from the joint Gambia Government / Medical Research Council (MRC) ethics committee and formal clearance was further obtained from the Hospital Management Board as required by the ethics committee. Informed consent was also obtained from the parents or legal guardians of all the patients.
Results
A total of 396 babies 257 (64.9%) males and 139 (35.1%) females) were admitted in the neonatal unit of the hospital during the study period. Of these, 105 (26.5%) satisfied the inclusion criteria and were enrolled in the study. Fifty-seven (54.3%) of the babies studied were males while 48 (45.7%) were females.
Fourteen (13.3%) of the babies studied had positive blood smears for malaria parasites (10 (71.4%) males and 4 (28.6%) females) with a male: female ratio of 2.5:1. Plasmodium falciparum was the only species of malaria identified in all the babies and bacterial cultures of their blood, cerebrospinal fluid and urine yielded no pathogen.
Twenty-eight (26.7%), comprising 12 (42.9%) males and 16 (57.1%) females, had proven bacterial septicaemia on blood culture while one (1%), a male, had proven neonatal meningitis. Five (35.7%) of the babies with malaria had lowdensity parasitaemia (+), 7 (50%) medium density parasitaemia (++) while 2 (14.3%) had high-density parasitaemia (+++).
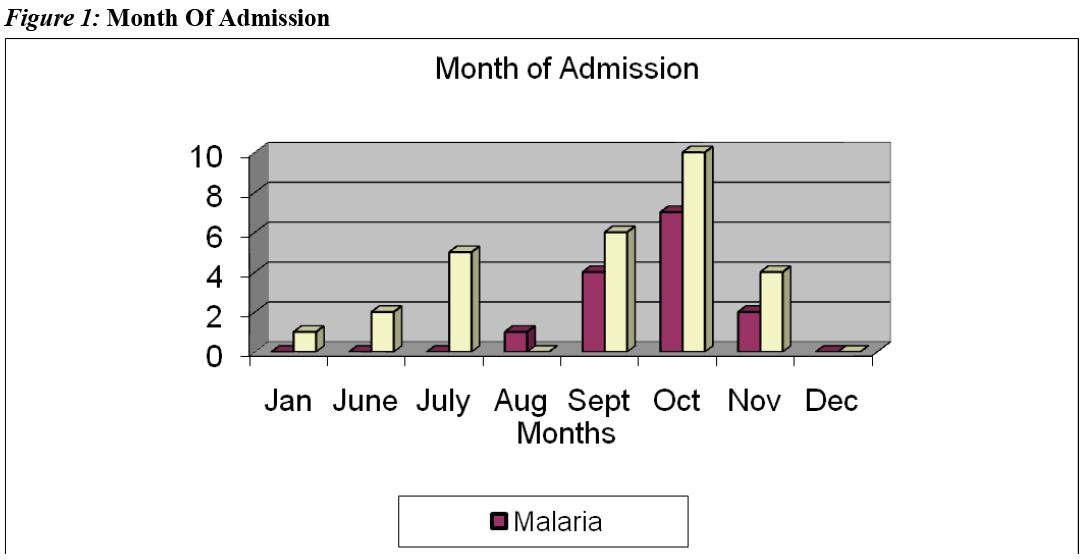
All the babies with malaria were admitted between the months of August and November. This period corresponds to the rainy season period in The Gambia during which there is peak malaria transmission in the country, "the malaria season".
Five (35.7%) of the babies with malaria were aged 0-7 days, 2 (14.3%) 8-14 days and 7 (50%) 15-28 days. From classification therefore, 5 (35.7%) of the babies had congenital malaria while (9) 64.3% had acquired malaria. None of the patients had received a blood transfusion and thus no case of transfusional malaria was seen.
The mean weight of the babies studied was 3.0 kg (1.4-4.7kg) while the babies with malaria had a mean weight of 3.0 kg (2.3-3.9kg). Only two (14.28%) of the babies with malaria weighed less than 2.5 kg.
The mean gestational age of the babies studied was 38 weeks (32.0 - 42.0 weeks) while the mean gestational age of the babies with malaria was 37 weeks (32.0-39.0 weeks), and only three of the babies with malaria had a gestational age of less than 37 weeks.
A greater proportion of the babies studied were from the urban area, 74 (70.5%) compared with 31 (29.5%) from the rural area. The babies with malaria were similarly distributed [urban 9 (64.3%), rural 5 (35.7%)]. No significant difference in the occurrence of malaria was observed between the urban and rural babies (P = 0.14).
Thirteen (92.9%) of the babies with malaria were delivered by the spontaneous vertex approach, 1 (7.1%) by elective lower segment caesarean section and none by instrumental delivery.
Of the babies with malaria, there were 5 (35.7%) primiparae, 7 (50%) multiparae and 2 (14.3%) grand multiparae.
Three (21.4%) of the babies with malaria were delivered at home without any trained person in attendance while 11 (78.6%) were delivered in a health facility with a trained midwife, at least, in attendance.
Only two mothers of the babies with malaria received pyrimethamine prophylaxis during pregnancy while 8 (57.1%) received anti-malarial treatment at least once during the pregnancy. The babies that had malaria did not differ from those who did not have malaria in terms of maternal anti-malarial treatment during pregnancy (P = 0.94).
The clinical features observed among the babies with malaria included: fever (12; 85.7%), refusal to feed / poor suck (11; 78.6%), maternal fever in the last trimester (9; 64.3%), excessive crying (8; 57.1%), difficult breathing (6, 42.9%), vomiting (6; 42.9%), hepatomegaly (6; 42.9%), abdominal distension (5; 35.7%), irritability (4; 28.6%), seizures (3; 21.4%), splenomegaly (3; 21.4%), pallor (3; 21.4%), depressed neonatal (primitive reflexes) (3; 21.4%), jaundice (2; 14.3%), and bleeding (1; 7.1%). These features were similarly distributed for the group that had proven bacterial septicaemia except for depressed neonatal reflexes. It was found that a statistically significant proportion of patients with proven bacterial septicaemia compared with patients with malaria had depressed neonatal (primitive) reflexes (P =0.01).
| N (%) | |||
|---|---|---|---|
| Age (Days) | Enrolled | Malaria | Bact. Sepsis |
| 0-7 | 55 (52.4) | 5 (35.7) | 16 (57.1) |
| 8-14 | 26 (24.8) | 2 (14.3) | 8 (28.6) |
| 15-28 | 24 (22.9) | 7 (50) | 4 (14.3) |
| Total | 105 | 14 | 28 |
Table 1: Age Distribution of the Babies
Discussion
Although the patients reported in this study were a highly select group being that they were all admitted in the neonatal unit, the prevalence rate of 13% obtained in the study suggests that neonatal malaria should no longer be considered as being uncommon in The Gambia. This is supported by other studies that show similarly high rates among neonates from other hyper endemic areas of tropical Africa.[13-15, 17, 21-23, 26, 29, 30]
The apparent resistance of newborns to malaria had been attributed to several factors such as the presence of immunity passively acquired from semi-immune mothers, a high percentage and relative resistance to parasitisation of foetal haemoglobin-containing erythrocytes, and a pamino benzoic acid-deficient milk diet of the neonate.[9, 10, 31, 32] This protection is now being shown to be anything but adequate, and Khan and Talibi (1972), maintain that immunity passively transferred to the newborn does not play a significant role in restricting the development of malaria parasites in the newborn.[33]
Three types of neonatal malaria have been described: congenital, acquired and transfusional. [18, 19, 21-23] Congenital malaria occurs when malaria parasites cross the placenta either during the process of normal pregnancy or at the time of delivery. [34, 35] This implies that malaria parasites may invade the placenta and subsequently be transmitted to the foetus. Ordinarily the placenta is thought to be an effective barrier against the passage of plasmodia and the cause of its breakdown allowing parasites to pass to the foetus is not known.36 Early workers considered mechanical damage to be important 37 while others considered parasite-induced pathological changes to be responsible. [38] Schwetz (1939), thought that acute fever caused greater friability of the placenta [39] while Tanner and Hewlett (1939), considered premature placental separation as an important factor.[40] The failure of pyrimethamine prophylaxis during pregnancy to fully protect babies from congenital malaria has also been reported by various workers. [26, 31, 41]
Acquired malaria on the other hand results from mosquito bites anytime after delivery, with asexual parasitaemia detected after the minimum incubation period of one week while neonatal transfusional malaria is said to occur when malaria parasites are detected in a neonate, whose peripheral blood film was previously negative, after a blood transfusion.[42] The mean interval between blood transfusion and the presence of symptoms is said to be three days.[43]
A higher proportion of the patients with malaria in this study were aged more than one week. From the foregoing, this tends to suggest that most of the infections were acquired rather than congenital. McGregor (1984), had shown that in The Gambia congenitally acquired malaria is not a common occurrence and that primary patent parasitaemia is usually postnatally acquired.[44] This differs from the predominantly congenital malaria seen in the series by Ibhanesebhor[43] (1995) and a preponderance of transfusional malaria in the study by Thapa et al.(1987)[45]
None of the babies recruited in this study had had a blood transfusion and that explains the absence of transfusional neonatal malaria in this series.
All the babies with malaria in this study were admitted between the months of August and November. This period corresponds to the rainy season in The Gambia during which there is peak malaria transmission in the country. McGregor (1984) made a similar observation in which detectable parasitaemia in infants aged less than three months was substantially more frequent during the wet season than in dry season.[44] Similar observations were made in Ghana [46] and Nigeria.[47]
A male preponderance was observed in this study. This differs from the series by Ibhanesebhor (1995) Akindele et al. (1993) and Thapa et al. (1987) [26,43,45] in which no sex predilection was reported.
Most of the babies with malaria in this study had medium or low-density parasitaemia. Ibhanesebhor (1995) Akindele et al. (1993) showed a similar trend.[26, 43] McGuiness et al. (1998) and Wagner et al. (1998) also found low-density parasitaemia in neonates and infants, respectively.[46, 48] Greenwood et al.(1991) had argued that the level of parasitaemia must be above a certain level before the diagnosis of clinical malaria can be made, [4] but this may not necessarily apply to neonates and young infants.
The babies with malaria described in this study were all symptomatic. This is contrary to the previously held belief that symptomatic malaria is rare during the neonatal period. Symptomatic neonatal malaria has also been described in several other studies.[18-23, 43]
In terms of symptomaology, 85% of the patients with neonatal malaria in this study presented with fever, while a significant number presented with refusal to feed, excessive crying, vomiting, abdominal distension, difficult breathing and hepatomegaly. These features were also similarly observed in the patients with proven bacterial septicaemia. Similar findings were made by other workers.[18-23,43] It was further observed that depressed primitive (neonatal) reflexes were significantly less frequent among patients with neonatal malaria compared with patients that had proven bacterial septicaemia. The explanation for this finding is not clear but it is possible that involvement of the central nervous system in patients with neonatal septicaemia may explain the higher occurrence of depressed primitive reflexes in this category of patients.
This study was conducted in 1999, about a decade ago, but to the best of our knowledge there is no more recent study from The Gambia on the subject and more recent studies from the sub-region and beyond present results akin to what we obtained.[13-16]
This study has demonstrated the following findings: That neonatal malaria is not uncommon at the Royal Victoria Hospital in Banjul, The Gambia neonatal malaria is more common in babies more than one week of age and during the rainy season. The clinical features of neonatal malaria are not distinguishable from those of neonatal septicaemia; however depression of primitive reflexes was more common in neonates with septicaemia.
Acknowledgment
The following persons are gratefully acknowledged: Drs. G C Enwere, Mariatou Jallow, S Usen, R Adegbola, S Allen, M Ota and I Ike: Messrs K O Jaiteh, Peters, Lemon and all the staff of the Paediatric Unit of the Royal Victoria Hospital, Banjul.
References
- White NJ. Malaria. In: Cook GG (Ed). Manson's Tropical Diseases 20th ed. London: Edward Arnold; 1996; 1087-1164.
- Bruce-Chwatt LJ. History of malaria from prehistory to eradication In: Wernsdoofer WH and McGregor I (Eds). Principles and Practice of Malariology Edinburgh: Churchill Livingstone; 1988; 1-59.
- Samba EM. The burden of malaria in Africa. Afr Health 1997; 19(2): 17
- Greenwood B, Marsh K and Snow R. Why do some African children develop severe malaria? Parasitol Today 1991; 7: 277-281.
- Sturchler D. How much malaria is there worldwide? Parasitol Today 1989; 5: 39-40.
- World Bank. World development report: investing in health. Oxford; 1993.
- Hensbroek MB. Cerebral malaria in children: pathogenesis, treatment Netherlands 1996; 11-7.
- Greenwood BM, Bradley AK, Greenwood AM, Byass P, Jammeh K and Marsh K. Mortality and morbidity from malaria among children in a rural area of The Africa. Trans R Soc Trop Med Hyg 1987; 81: 478-486.
- Hawking F. Milk, p-amino benzoate and malaria of rats and monkeys. Br Med J 1965; 1: 425-429.
- Wilson RJ, Pasvol G and Weatheral DJ. Invasion and Growth of Plasmodium falciparum in different types of human erythrocytes. Bull World Health Org 1977; 55: 179-186.
- Achidi EA, Perlman H and Salimou LS. Longitudinal study of seroreactivities to plasmodium falciparum antigen in Nigerian infants during their first year of life. Act Trop 1995; 59(2): 173-183.
- Egwunyega OA, Ajayi JA and Duhlinska- Popova DD. Transplacental passage of plasmodium falciparum and seroevaluation of newborns in Northern Nigeria. J Commun Dis 1995; 27(2): 77-83.
- de Pontual L, Poilane I, Ledeur F, Haouchine D, Lachassinne E, Collignon A et al. Report of a case of congenital malaria in France. J Trop Ped 2006; 52(6): 448-450.
- Mukhtar MY, Lesi, FEA, Iroha EU, Egri- Okwaji MTC and Mafe AG. Congenital malaria among inborn babies in a tertiary centre in Lagos, Nigeria. J Trop Ped 2006; 52 (1): 19-23.
- Nweneka CV and Eneh AU, Malaria parasitemia in neonates in Port-Harcourt, Nigeria. J Trop Ped 2004; 50 (2): 114- 116.
- Hewson MP, Simmer K and Blackmore T. Congenital malaria in a preterm infant. Journal of Pediatrics and Child Health 2003; 39 (9): 713-715.
- Ibhanesebhor SE and Okolo AA. Malaria parasitemia in neonates with predisposing risk factors for neonatal septicemia. Ann Trop Pediatric 1992; 12(3): 297-302.
- Heenequin C and Bouree P. Paludisme de la femme gestante et du nouveeau-ne. Bulletin de la Societe de Pathologie Exotique 1991; 84(5): 465-470.
- Boulos M, Barata L C and Amata-Neto Vl. Malaria congenita: descricao de u caso e consideracoes sobre o assunto. Revista do Hospital das Clinicas; Faculdade de Medicina Da Universidade de Sao Paulo 1993; 48(3): 127-129.
- Airede AL. Congenital malaria with chloroquine resistance. Ann Trop Pediatric 1991; 13(3): 273-276.
- Fischer PR. Congenital malaria: an African survey. Clin Pediatr 1997; 36(7): 411-413.
- McGregor IA. Congenitally acquired malaria. Postgrad Doc Afr 1986; 8: 52-56.
- Sodeinde O and Dawodou AH. Neonatal transfusional malaria: a growing clinical problem. Nig J Pediatric 1985; 12: 57-60.
- Logie DE, McGregor IA. Acute malaria in newborn infants. Br Med J 1970; 3: 404- 405.
- Lwanga SK and Lemeshow S. Sample size determination in health studies. Geneva World Health Organization 1991: 25
- Akindele JA, Sowunmi A and Abohweyere EJ. Congenital malaria in a hyper endemic area: a preliminary study. Ann Trop Pediatric 1993; 13(3): 273-276.
- Dubowitz LM, Dubowitz V and Goldberg C. Clinical assessment of gestational age in the newborn Infant. J Pediatric 1970; 77: 1.
- Etienne L. Identification of malaria parasites. In: Cheesbrough M and Prescott LM (Eds). Manual of Basic Techniques For a Health Laboratory. Geneva: World Health Organization; 1980: 166.
- Uneke C. Congenital Plasmodium falciparum malaria in sub-Saharan Africa; a rarity or frequent occurrence? Parasitol Research 2007; 101(4): 835-842.
- Sotimehin SA, Runsewe-Abiodun T, Oladapo OT, Njokanma OlF and Olanrewaju DM. Possible Risk Factors for Congenital Malaria at a Tertiary Care Hospital in Sagamu, Ogun State, South-West Nigeria. J of Trop Ped 2008; 54(5): 313-320.
- Nahlen BL, Akintunde A, Alakija T, Nguyen- Dinh P, Ogunbode O. Edungbola LD, et al. Lack of efficacy of pyrimethamine prophylaxis in pregnant Nigerian women. Lancet 1989; 2: 830-834.
- Ibeziako PA and Williams AIO. The effect of malaria chemoprophylaxis on immunoglobulin levels of pregnant Nigerian women and their newborns. Br J Obstet Gynecol 1980; 87: 976- 982.
- Khan AQ and Talibi SA. Epidemiological assessment of malaria transmission in an endemic area of East Pakistan and significance of congenital immunity. Bull World Health Organization 1972; 46: 783-792.
- Brabin BJ. Malaria in pregnancy, it's importance and control. Postgrad Doct Afr 1989; 11: 57-59.
- Mengert WF, Rights CR, Wash T and Bates CR. Placental transmission of erythrocytes. Am J Obstet Gynecol 1966; 69: 678-685.
- Marshall DE. The transplacental passage of malaria parasites in the Solomon Islands. Trans R Soc Trop Med Hyg 1983; 77(4): 470-473.
- Clark HC. The diagnostic value of the placental blood film in aestivo-automnal malaria. J Exp Med 1915; 22: 427-445.
- Wickramasuriya GAM. Some observations on malaria occurring in association with pregnancy with special reference to transplacental passage of parasites from maternal to fetal circulation. J Obstet Gynecol 1935; 42: 816-834.
- Schwetz J. Considerations sur la paludisme congenital. Bull Societe de Pathologie exotique 1939; 32: 44-50.
- Tanner NC and Hewlett RFL. Congenital malaria with report of a case in one of the twins. Lancet 1939; 2: 369-370.
- Olarewaju WI. Malaria in the neonate: report of 2 cases. West Afr J Med 1999; 18: 139-140
- Ibhanesebhor SE. The management of neonatal malaria. Postgrad Doc Afr 1996; 18(2): 38-39.
- Ibhanesebhor SE. Clinical characteristics of neonatal malaria. J Trop Pediatric 1995 41(6): 330-333.
- McGregor IA. Epidemiology, Malaria and Pregnancy. Am J Trop Med Hyg 1984; 33(4): 517-525.
- Thapa BR, Narang A and Bhakoo ON. Neonatal malaria: a clinical study of neonatal and transfusional malaria. J Trop Pediatr 1987; 33: 266-269.
- Wagner G, Koram K, McGuinness D, Bennett S, Nkrumah F and Riley E. High incidence of asymptomatic malaria infections in a birth cohort of children less than one year of age in Ghana, detected by multicopy gene polymerase chain reaction. Am J Trop Med Hyg 1998; 59(1): 115-123.
- Bruce-Chwatt LJ. Malaria in infants and children in Southern Nigeria. Ann Trop Med Parasitol 1952; 46: 173-200.
- McGuinness D, Koram K, Bennett S, Wagner G, Nkrumah F and, Riley E. Clinical case definitions for malaria: clinical malaria associated with very low parasite densities in African infants. Trans R Soc Trop Med Hyg 1998; 92: 527-531.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.