Non-invasive Diagnostic Accuracy of Transvaginal Ultrasound in Patients with Bowel Endometriosis: Systematic Review and Meta-analysis
Citation: Alyami HS. Risk of Dental Caries and Breastfeeding: A Systematic Review and Meta-analysis. Ann Med Health Sci Res. 2021;11: 1183-1187.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Background: Concerning endometriosis transvaginal sonography (TVS) is a quite sensitive tool for the detection of ovarian endometriomas and is a long way superior to routine clinical examination alone. Aim: This work aims to determine the accuracy, sensitivity, and specificity of non-invasive transvaginal ultrasound in bowel endometriosis patients. Materials and Methods: A systematic search was performed over different medical databases to identify Obstetrics & Gynecology studies, which studied the accuracy, sensitivity, and specificity of non-invasive transvaginal ultrasound in bowel endometriosis patients. Using the meta-analysis process, either with fixed or random-effects models, we conducted a meta-analysis on diagnostic accuracy (AUC) as a primary outcome, and sensitivity and specificity as secondary outcomes. Results: Eight studies were identified involving 1119 patients. The meta-analysis process revealed a pooled accuracy of 94.8%, a pooled sensitivity of 85.4%, and a pooled specificity of 98.1% (p < 0.01 respectively). Conclusion: To conclude, Transvaginal ultrasonography (TVUS) has become the primary diagnostic tool in the diagnosis of deep infiltrating pelvic endometriosis. In the majority of cases, TVUS will give enough information to the surgeon allowing for adequate preoperative planning without the need for magnetic resonance imaging (MRI). Compared to MRI, TVUS is widely available in gynecologic outpatient clinics, well-tolerated, less time-consuming, and less expensive.
Keywords
Transvaginal Ultrasound; Bowel Endometriosis; Diagnostic Accuracy
Introduction
Bowel endometriosis is one of the most severe forms of deep endometriosis. It causes pain and several intestinal complaints which include constipation, diarrhea, intestinal cramping, stomach bloating, feeling of incomplete evacuation, the passage of mucus, and rectal bleeding all through the menstrual length. A correct analysis of rectosigmoid endometriosis permits offering to the affected person both a hormonal or surgical treatment. moreover, amongst sufferers requiring surgical procedures, the characteristics of the rectosigmoid endometriosis (together with the size of the nodules and presence of multifocal disease) allow to preoperatively predict the type of surgical procedures (shaving, disk resection, or segmental bowel resection). [1]
Endometriosis is prime gynecological health trouble being associated with infertility, chronic pelvic ache, and dysmenorrhea with an estimated incidence of 6.1% of women of reproductive age. The presence of endometrial tissue, fibrosis, and hyperplasia under the peritoneum is described as deep infiltrating endometriosis (DIE) which debts for about 15% to 30%of all recognized endometriosis cases. The maximum common sites of the DIE had been reported to be uterosacral ligaments, the rectosigmoid colon, the vagina, and the bladder. [2]
Over the past decade, the usage of transvaginal sonography (TVS) has improved the best non-invasive evaluation of patients with suspected pelvic pathologies. Concerning endometriosis, TVS is a quite sensitive tool for the detection of ovarian endometriomas and is a long way superior to routine clinical examination alone. [3]
The gold standard for the diagnosis of endometriosis has traditionally been the visualization of endometriosis through laparoscopy. Studies comparing the pelvic US to laparoscopy for the prognosis of DIE have produced varying results. [4]
This work aims to determine the accuracy, sensitivity, and specificity of non-invasive transvaginal ultrasound in bowel endometriosis patients.
Literature Review
Our review came following the (PRISMA) statement guidelines. [5]
Study eligibility
The included studies should be in English, a journal published article, and a human study describing bowel endometriosis patients. The excluded studies were non-English, or animal studies, or describing other types of endometriosis patients (e.g uterine endometriosis, or describing other diagnostic modalities (e.g. Computed Tomography).
Study identification
Basic searching was done over the PubMed, Cochrane library, and Google scholar using the following keywords: Transvaginal Ultrasound, Bowel Endometriosis, and diagnostic Accuracy.
Data extraction and synthesis
RCTs, clinical trials, and comparative studies, which studied the accuracy, sensitivity, and specificity of non-invasive transvaginal ultrasound in bowel endometriosis patients, will be reviewed.
Outcome measures included diagnostic accuracy (AUC) as a primary outcome and sensitivity and specificity as a secondary outcome.
Study selection
We found 190 records, 115 excluded based on title and abstract review; 75 articles are searched for eligibility by full-text review; 30 articles cannot be accessed; 14 studies were reviews and case reports; 17 were not describing bowel endometriosis; the desired diagnostic modality not used in 6 studies leaving 8 studies that met all inclusion criteria.
Statistical methodology
After the pooling of data, Proportions (%), with 95% confidence intervals (CI) were calculated, using MedCalc statistical software (Belgium). After the Q test of heterogeneity, the I2- statistics (either the fixed-effects model or the random-effects model) were done within the meta-analysis process.
Results
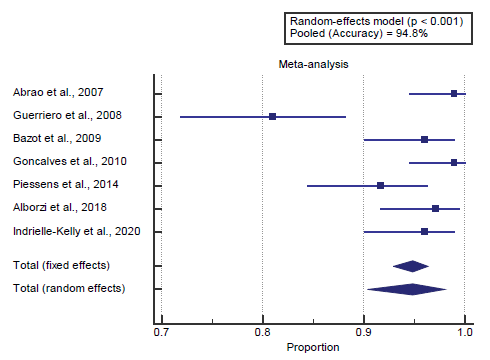
The included studies were published between 2007 and 2020. Regarding the type of included studies, all 8 studies were prospective [Table 1]. [6-12]
| N | Author | Type of study |
Number of patients | Age (average years) |
Reference standard |
|---|---|---|---|---|---|
| Total | |||||
| 1 | Abrao et al. [6] | Prospective | 104 | 33.8 | Histology |
| 2 | Guerriero et al. [7] | Prospective | 88 | 33 | Histology |
| 3 | Bazot et al. [8] | Prospective | 92 | 31.8 | Histology |
| 4 | Goncalves et al. [9] | Prospective | 194 | 34.2 | Histology |
| 5 | Fratelli et al. [10] | Prospective | 190 | 35 | Surgery and histology |
| 6 | Piessens et al. [11] | Prospective | 85 | -- | Histology |
| 7 | Alborzi et al. [12] | Prospective | 317 | 31 | Histology |
| 8 | Indrielle-Kelly et al. [13] | Prospective | 49 | 32.4 | Surgery and histology |
| #Studies arranged via publication year. | |||||
Table 1: Patients and study characteristics.
Regarding patients’ characteristics, the total number of patients in all the included studies was 1119 patients, while their average age was (33) years [Table 1].
The reference standard diagnostic method was histopathology in all studies, with 2 studies confirmed the diagnosis with an additional surgical confirmation.
A meta-analysis study was done on 8 studies that described diagnostic accuracy, sensitivity, and specificity; with an overall number of patients (N=1119) [Table 2]. [6-12]
| N | Author | Primary outcome | Secondary outcomes | |
|---|---|---|---|---|
| Accuracy | Sensitivity | Specificity | ||
| % | % | % | ||
| 1 | Abrao et al. [6] | 99 | 98 | 100 |
| 2 | Guerriero et al. [7] | 81 | 67 | 92 |
| 3 | Bazot et al. [8] | 96 | 94 | 100 |
| 4 | Goncalves et al. [9] | 99 | 98 | 100 |
| 5 | Fratelli et al. [10] | -- | 66 | 96 |
| 6 | Piessens et al. [11] | 91.7 | 88 | 93.3 |
| 7 | Alborzi et al. [12] | 97.16 | 88.46 | 98.87 |
| 8 | Indrielle-Kelly et al. [13] | 96 | 67 | 100 |
Table 2: Summary of outcome measures in all studies.
Each outcome was measured by:
Pooled Proportions (%)
• For pooled accuracy.
• For pooled sensitivity.
• For pooled specificity.
Concerning the primary outcome measure, we found 7 studies reported accuracy with a total number of patients (N=929). I2 (inconsistency) was 82% with a highly significant Q test for heterogeneity (p<0.0001), so random-effects model was carried out; with pooled accuracy=94.8% (95% CI=90.36 to 97.99). Using the random-effects model, the meta-analysis process revealed a pooled accuracy of 94.8% (p<0.01) [Figure 1].
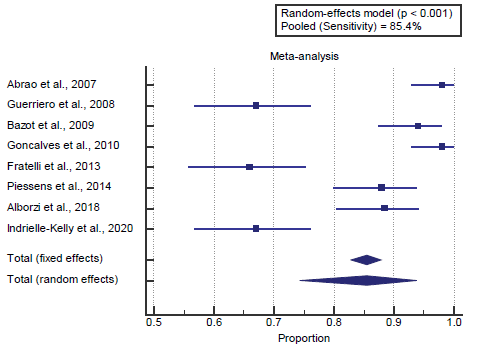
Concerning the secondary outcome measures, we found 8 studies reported sensitivity with a total number of patients (N=1119).
I2 (inconsistency) was 93.9% with a highly significant Q test for heterogeneity (p<0.0001), so random-effects model was carried out; with pooled sensitivity=85.4% (95% CI=74.335 to 93.768). Using the random-effects model, the meta-analysis process revealed a pooled sensitivity of 85.4% (p<0.01) [Figure 2].
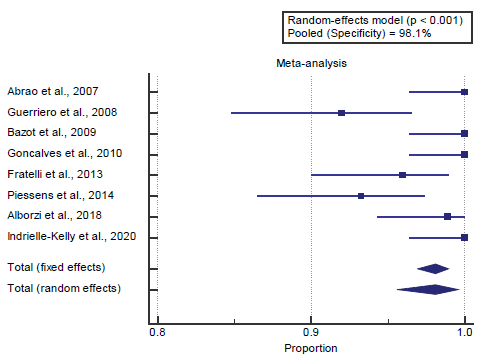
We found 8 studies reported specificity with a total number of patients (N=1119).
I2 (inconsistency) was 77.9% with a highly significant Q test for heterogeneity (p<0.0001), so random-effects model was carried out; with pooled specificity=98.1% (95% CI=95.606 to 99.587). Using the random-effects model, the meta-analysis process revealed a pooled specificity of 98.1% (p<0.01) [Figure 3].
Discussion
This work aims to determine the accuracy, sensitivity, and specificity of non-invasive transvaginal ultrasound in bowel endometriosis patients. The included studies were published between 2007 and 2020. Regarding the type of included studies, all 8 studies were prospective.
Regarding patients’ characteristics, the total number of patients in all the included studies was 1119 patients, while their average age was (33) years. The reference standard diagnostic method was histopathology in all studies, with 2 studies confirmed the diagnosis with an additional surgical confirmation.
A meta-analysis study was done on 8 studies that described diagnostic accuracy, sensitivity, and specificity; with an overall number of patients (N=1119). Concerning the primary outcome measure, we found 7 studies reported accuracy with a total number of patients (N=929).
Using the random-effects model, the meta-analysis process revealed a pooled accuracy of 94.8% (p<0.01). This came in agreement with Alborzi et al. [2] and Ferrero et al. [1]
Alborzi et al. reported that we assessed >500 sufferers for eligibility of whom 317 (simplest non-virgins) had been enrolled in this study through consecutive sampling technique. The mean age of the subjects changed to 31±5.4 (ranging from 19 to 49). The analysis of DIE turned into showed in 252 (79.5%) patients. these 252 patients had a general range of 350 DIE lesions in different locations. The baseline characteristics of the patients, as well as the laparoscopic and histopathological, the diagnostic accuracy of TVS, were 82.33. [2]
Ferrero et al. reported that the most popular practice is to perform TVS without BP, it may be theoretically advocated that the usage of BP may improve the accuracy of the technique; therefore, we decided to use a non-inferiority study design. they primarily based our sample size calculation at the accuracy calculated from a lately posted meta-analysis 30 including most effective the research investigating the role none enhanced TVS with BP (98.9%) 9, 10. We expected that the accuracy turned into identical among TVS with and without BP. We calculated that 262 sufferers (undergoing TVS with BP, TVS without BP, and laparoscopy) had been required to offer 95% energy to expose the non-inferiority of TVS without BP, with a noninferiority margin of 3%. [1] Concerning the secondary outcome measures, we found 8 studies reported sensitivity with a total number of patients (N=).
Using the random-effects model, the meta-analysis process revealed a pooled sensitivity of 85.4% (p<0.01) [Figure 2]. Which came in agreement with Chowdary et al., [13] Alborzi et al., [2] Van den Bosch & Van Schoubroeck, [14] and Guerriero et al. [15]
Chowdary et al. reported that Seventy-nine percent (42/53) of the patients had laparoscopic findings consistent with their ultrasound findings (P<0.0001). Detection of thickened pericolic fat was most associated with mild-moderate endometriosis at the time of laparoscopy. Uterosacral ligament thickening (sensitivity 0.62, specificity 0.73, area under the ROC curve 0.67, P<0.05). [13]
Alborzi et al., reported that, of the 317 patients, 245 (77.three%) had DIE lesions on TVS examination, and 210 of them had positive pathologic findings (PPV=85.7%). a complete of 72 ladies confirmed regular findings on the TVS exam, and 30 of them had ordinary pathology (NPV=41.6%). however, 42 of the 72 women with normal TVS examinations had pathologic abnormalities. The sensitivity and specificity of TVS for the prognosis of DIE had been 83.3% and 46.1%, respectively. [2]
Van den Bosch & Van Schoubroeck reported that the diagnostic accuracy for DIE was evaluated for 3 locations: the rectosigmoid, the recto-vaginal septum, and the uterosacral ligament. They confirmed that the general diagnostic performance turned into comparable for both strategies. For DIE inside the rectosigmoid, the pooled sensitivity for MRI and TVUS was 0.85 and 0.85respectively, whilst the pooled specificity was 0.95 and 0.96, respectively. For DIE in the rectovaginal septum, the pooled sensitivity for MRI and TVUS was zero.85, and zero.85, respectively, even as the pooled specificity was 0.95 and 0.96, respective. [14]
Guerriero et al. reported that our extended search identified a total of 801 citations, among which 19 studies (n=2639) were considered eligible and included in the meta-analysis. Overall pooled sensitivity, specificity, positive likelihood ratio (LR+), and negative likelihood ratio (LR–) of TVS for detecting DIE in the rectosigmoid were 91%, 97%, 33.0, and 0.10, respectively. Significant heterogeneity was found for sensitivity (I2, 90.8%; P<0.001) and specificity (I2, 76.8%; P<0.001). [15]
We found 8 studies reported specificity with a total number of patients (N=1119). Using the random-effects model, the meta-analysis process revealed a pooled specificity of 98.1% (p<0.01). Which came in agreement with Young et al., [16] Turocy & Benacerraf [4] and Hudelist et al. [3]
Young et al. reported that, of 117 patients referred for TVUS-BP, 113 had completed exams. 57 of 113 patients underwent surgical exploration within one year and DE 44 was identified surgically in 23. DE of the rectosigmoid colon and/or rectovaginal septum was detected 45 with a sensitivity of 94% and specificity of 100%. DE of the retro cervical 46 regions and/or uterosacral ligaments was detected with a sensitivity of 86% and specificity of 94%. [16]
Turocy & Benacerraf reported that TVUS has been reported as comparable, if not superior, to MRI when evaluating women with pelvic pain. For rectosigmoid endometriosis.TVUS had a sensitivity and specificity of 98% and 100%, respectively, compared with 83% and 98% for MRI. [4]
Hudelist et al. reported that, the prevalence of bowel endometriosis various from 24 to 73.3%. LR+ ranged from 4.8 to 48.56 and LR−ranged from 0.02 to 0.36, with huge self-assurance intervals. Pooled estimates of sensitivities and specificities had been 91% and 98%; LR+ and LR− were 30.36 and 0.09, and fine and negative predictive values were 98% and 95% respectively. 3 of the research used bowel preparations to enhance the visibility of the rectal wall; one examines at once in comparison the usage of water evaluation vs. no previous bowel enema, for which the LR− was 0.04 and 0.47, respectively. [3]
Conclusion
To conclude, transvaginal ultrasonography (TVUS) has become the primary diagnostic tool in the diagnosis of deep infiltrating pelvic endometriosis. In the majority of cases, TVUS will give enough information to the surgeon allowing for adequate preoperative planning without the need for magnetic resonance imaging (MRI). Compared to MRI, TVUS is widely available in gynecologic outpatient clinics, well-tolerated, less timeconsuming, and less expensive.
Competing Interests
The authors declare that they have no competing interests. All the listed authors contributed significantly to the conception and design of study, acquisition, analysis, and interpretation of data and drafting of the manuscript, to justify authorship.
REFERENCES
- Ferrero S, Scala C, Stabilini C, Vellone VG, Barra F, Leone Roberti Maggiore U. Transvaginal sonography with vs without bowel preparation in diagnosis of rectosigmoid endometriosis: prospective study. Ultrasound Obstet Gynecol. 2019;53:402-409.
- Alborzi S, Rasekhi A, Shomali Z, Madadi G, Alborzi M, Kazemi M, et al. Diagnostic accuracy of magnetic resonance imaging, transvaginal, and transrectal ultrasonography in deep infiltrating endometriosis. Medicine 2018;97.
- Hudelist G, English J, Thomas AE, Tinelli A, Singer CF, Keckstein J. Diagnostic accuracy of transvaginal ultrasound for non-invasive diagnosis of bowel endometriosis: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2011;37:257-263.
- Turocy JM, Benacerraf BR. Transvaginal sonography in the diagnosis of deep infiltrating endometriosis: a review. J Clin Ultrasound. 2017;45:313-318.
- Liberati A, Altman D, Tetzlaff J, Mulrow C, Gøtzsche P, Ioannidis J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions. Bmj 2009;339.
- Abrao MS, Gonçalves MO da C, Dias Jr JA, Podgaec S, Chamie LP, Blasbalg R. Comparison between clinical examination, transvaginal sonography and magnetic resonance imaging for the diagnosis of deep endometriosis. Hum Reprod. 2007;22:3092-3097.
- Guerriero S, Ajossa S, Gerada M, Virgilio B, Angioni S, Melis GB. Diagnostic value of transvaginal ‘tenderness-guided’ultrasonography for the prediction of location of deep endometriosis. Hum Reprod. 2008;23:2452-2457.
- Bazot M, Lafont C, Rouzier R, Roseau G, Thomassin-Naggara I, Daraï E. Diagnostic accuracy of physical examination, transvaginal sonography, rectal endoscopic sonography, and magnetic resonance imaging to diagnose deep infiltrating endometriosis. Fertil Steril. 2009;92:1825-1833.
- Goncalves MO da C, Podgaec S, Dias Jr JA, Gonzalez M, Abrao MS. Transvaginal ultrasonography with bowel preparation is able to predict the number of lesions and rectosigmoid layers affected in cases of deep endometriosis, defining surgical strategy. Hum Reprod. 2010;25:665-671.
- Fratelli N, Scioscia M, Bassi E, Musola M, Minelli L, Trivella G. Transvaginal sonography for preoperative assessment of deep endometriosis. J Clin Ultrasound. 2013;41:69-75.
- Piessens S, Healey M, Maher P, Tsaltas J, Rombauts L. Can anyone screen for deep infiltrating endometriosis with transvaginal ultrasound? Aust N Z J Obstet Gynaecol. 2014;54:462-468.
- Indrielle-Kelly T, Frühauf F, Fanta M, Burgetova A, Lavu D, Dundr P, et al. Diagnostic Accuracy of Ultrasound and MRI in the Mapping of Deep Pelvic Endometriosis Using the International Deep Endometriosis Analysis (IDEA) Consensus. BioMed Res Int. 2020;2020.
- Chowdary P, Stone K, Ma T, Readman E, McIlwaine K, Druitt M, et al. Multicentre retrospective study to assess diagnostic accuracy of ultrasound for superficial endometriosis—Are we any closer? Aust N Z J Obstet Gynaecol. 2019;59:279-284.
- Van den Bosch T, Van Schoubroeck D. Ultrasound diagnosis of endometriosis and adenomyosis: State of the art. Best Pract Res Clin Obstet Gynaecol. 2018;51:16-24.
- Guerriero S, Ajossa S, Orozco R, Perniciano M, Jurado M, Melis GB, et al. Accuracy of transvaginal ultrasound for diagnosis of deep endometriosis in the rectosigmoid: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2016;47:281-289.
- Young SW, Dahiya N, Patel MD, Abrao MS, Magrina JF, Temkit M, et al. Initial accuracy of and learning curve for transvaginal ultrasound with bowel preparation for deep endometriosis in a US tertiary care center. J Minim Invasive Gynecol. 2017;24:1170-1176.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.