Nutraceutical effects of Mediterranean citrus extracts in dysglycemia: a pilot study
2 Diabetes Unit, U.O.C. Territorial Pharmacy, ASL Roma 5, Tivoli, Italy
3 Metabolic and neuromuscular unit, "A. Meyer" Children's Hospital, University of Florence, Italy
Received: 05-Sep-2022, Manuscript No. AMHSR-22-76146; Editor assigned: 08-Sep-2022, Pre QC No. AMHSR-22-76146; Reviewed: 22-Sep-2022 QC No. AMHSR-22-76146; Revised: 29-Sep-2022, Manuscript No. AMHSR-22-76146; Published: 05-Oct-2022
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Background: Dysglycemia is a subclinical condition of altered glucose levels (intermediate hyperglycaemia), considered as a high-risk factor for diabetes and cardiovascular diseases. Lifestyle change, including diet and exercise, along with the management of blood glucose levels is the primary prevention of diabetes. Aim: In this clinical study, we investigated the potential role of a nutraceutical compound, based on Mediterranean citrus extracts, in regulating blood glucose in dysglycemic adults. Material and Methods: 40 adults with dysglycemia (100-125 mg/dl) were instructed to take a food supplement based on 460 mg Lemotrin® complex plus 100 µg chromium picolinate, 1 tablet twice a day during main meals for 12 weeks, in addition to general indications of a Mediterranean diet, maintaining overall constant dietary habits. Reduction of blood glucose levels was set as the primary endpoint, while reduction of HOMA index, insulinemia, body weight, abdominal circumference and improvement of the lipid profile as secondary endpoints. Results: After 12 weeks significant reductions of fasting blood glucose (-7.7%), fasting insulin (-31.6%), HOMA-index (-35.73%), glycated hemoglobin (A1c) (-4.78%) levels were observed, with a non-significant body weight reduction. Conclusion: Our findings suggest that the supplementation of the Mediterranean citrus complex improves the glycemic profile in subjects with dysglycemia.
Keywords
Dysglycemia; Fasting blood glucose; HbA1c; HOMA index; Mediterranean extract
Introduction
Dysglycemia is defined as an “altered state of hyperglycaemia with glucose levels below the diagnostic levels of diabetes”[1].The European guidelines, suggest a more appropriate term, such as "carbohydrate intolerance", to underline that not necessarily a subject will become diabetic, while the Italian guidelines AMD-SID (Associazione Medici Diabetologi -Società Italiana di Diabetologia) suggest to define precisely the various types of dysglycemia: impaired fasting glucose (IFG:100-125 mg/dl), impaired glucose tolerance 2 hours after oral glucose load (IGT:140-199 mg/dl) and glycated hemoglobin (HbA1c) values between 42 and 48 mmol/mol (6.00%-6.49%) [2]. According to the data published by the American Diabetes Prevention Program, 11% of people with dysglycemia develop type 2 diabetes within three years, while one in four patients within five years; in Europe, about 63 million have dysglycemia[3]. It is generally a clinically silent condition, associated with increased body weight, increased insulin resistance, and decreased insulin secretion due to a lower β-cell function; the worsening of these parameters is related to the progression to diabetes[4-6]. Dysglycemia is considered a risk factor not only for diabetes but also for cardiovascular diseases[7-9]. In patients with type 2 diabetes, an association has been shown between the degree of hyperglycaemia and increased risk of neuropathy, nephropathy, myocardial infarction, stroke, peripheral atherosclerotic disease, cardiovascular mortality, and all-cause mortality. A point (1%) reduction in the HbA1c level can reduce the risk of microvascular complications by more than 30% over time[10,11].
Insulin and glucagon are the two primary hormones that regulate the levels of glucose. An increased insulin secretion causes insulin resistance, an impaired sensitivity to insulin, making this hormone less effective; insulin resistance is associated with hypertension, thus prediabetic people may be more at risk to have altered blood pressure[12,13]. Thus, a tight control of glycemia and hypertension is mandatory in primary prevention[14]. An earlier diagnosis will allow earlier intervention, potentially reducing the onset of diabetes. Dietary recommendations are pivotal components of a correct lifestyle for prevention and treatment of type 2 diabetes and of the associated metabolic derangement (obesity, hypertension, dyslipidemia); however, it is difficult to follow them for a long term. Pharmacotherapy is necessary for patients not compliant or failing to reach the target blood glucose levels with the lifestyle modification alone[15,16]. However, adverse effects can occur when approaching the available drugs to lower blood glucose levels and improve insulin sensitivity. Therefore, an alternative way to modulate blood glucose profile may be the use of nutraceutical compounds , that are generally considered safe and free from significant side effects.
Aim of the study
Polyphenols are a class of plant compounds with various antioxidant and anti-inflammatory actions: They play an important protective role in metabolic and cardiovascular disease[17].
In this observational pilot study, we reported our real life clinical experience on the potential role of a nutraceutical compound, based on Mediterranean extracts from Citrus, in regulating blood glucose levels in dysglycemic people.
Secondary outcomes were to evaluate the effect of the compound on lipid profile and nutritional status (weight, body mass index and waist circumference).
Materials and methods
In this observational real life pilot study, 40 outpatients with Impaired Fasting Glucose levels (IFG) were screened and enrolled. They were in follow up at diabetes unit of S. Camillo hospital, Rome.
The patients were extracted from the database smartdigitalclinic ® (Meteda, Italy) among those with the last visit in the previous 12 months. Inclusion criteria were: Men and women with an age of 40-70 years and dysglycemia (fasting blood glucose levels=100-125 mg/dL and HbA1c=6,00-6,49%) on at least two consecutive occasions. Dysglycemia was recognized and diagnosed within the last 2 years. In previous visits patients were evaluated from a nutritional point of view: All received counselling about lifestyle changes, including increase of physical activity; a personalized diet was prescribed according to Mediterranean pattern; overweight ones had a hypocaloric prescription (20-25 kcal/kg), normal weight had 25-30 kcal/kg. Proteins were 1.0-1.1 g/kg/day with a particular recommendation to include plant foods (legumes, pulses) 2-3 times a week; carbohydrate were 40%-50% of total caloric intake, fiber 15 g/1000 kcal; fats were about 30% with high monounsatured/satured ratio; a moderate sodium restriction was recommended in hypertensive patients.
The subjects were telephonically invited to be reevaluated; in the first visit (T0) those with unchanged or worsened laboratory tests notwithstanding the adherence to lifestyle intervention program were enrolled (T0). Exclusion criteria were: Hyperglycemia secondary to pathological or iatrogenic causes, systemic diseases associated with hyperglycemia, current or previous diagnosis of diabetes (Type 1 or Type 2), uncompensated dysthyroidism, treatment with drugs potentially interacting with glucose metabolism (e.g. corticosteroids, atypical antipsychotics), currently pregnant or planning to become pregnant in the subsequent 12 weeks, recent myocardial infarction (less than 6 months), vascular surgery or stroke within the past year, allergy to one of the components of the food supplement. The participation to the study was voluntary, they all were adequately informed of the aims, methods, clinical evaluation details, and signed an informed consent. The study was performed at the Diabetology department, San Camillo Forlanini Hospital, Rome, in accordance with the current legislation and the declaration of Helsinki.
The diet and the current pharmacological therapy (e.g. anthypertensive, acetylsalycilic acid, fibrates) was not changed; no other nutraceutical supplementation was taken.
All subjects were instructed to take a food supplement based on Mediterranean extracts (460 mg Lemotrin® complex: 20% polyphenols from Citrus limon Risso fruit extract (Eriocitrin, Hesperidin, Narirutin), 28% hesperidin and other polyphenols (Didymine, Narirutin) from Citrus Sinensis L. Osbeck fruit extract) and anthocyanins, plus 100 µg chromium picolinate (the product was provided by Esserre Pharma, Rome, Italy): 1 tablet 2 times a day during main meals for 12 weeks, in addition to a recommended Mediterranean diet, maintaining overall constant dietary habits. the nutraceutical compound was given free. All the patients were evaluated after 12 weeks (t1): during the visit body weight, blood pressure, diet and therapy adherence were checked. Parameters detected were: Fasting blood glucose, Glycosylated hemoglobin (HbA1c), fasting insulinemia, HOMA index, body weight, abdominal circumference, Total Cholesterol (TC), Low-Density Lipoprotein Cholesterol (LDL-c), High-Density Lipoprotein Cholesterol (HDL-c), Non-High-Density Lipoprotein Cholesterol (Non-HDL-c) and Triglycerides (TG).
The Primary endpoint was the improvement of the glucose metabolism parameters (fasting glycemia and HbA1c). Secondary endpoints were: reduction of HOMA index, fasting insulinemia, body weight, abdominal circumference and improvement of the lipid profile (Total Cholesterol (TC), Low-Density Lipoprotein Cholesterol (LDL-c), High-Density Lipoprotein Cholesterol (HDL-c), Non-High-Density Lipoprotein Cholesterol (non-HDL-c) and Triglycerides (TG)).
Statistical analysis
To evaluate the effect of the product in the single group, One-way repeated measures ANOVA test was used. A significance level of <0.05 was considered. Analyses were performed using Free Statistics Calculators version 4.0 © 2006-2022.
Results
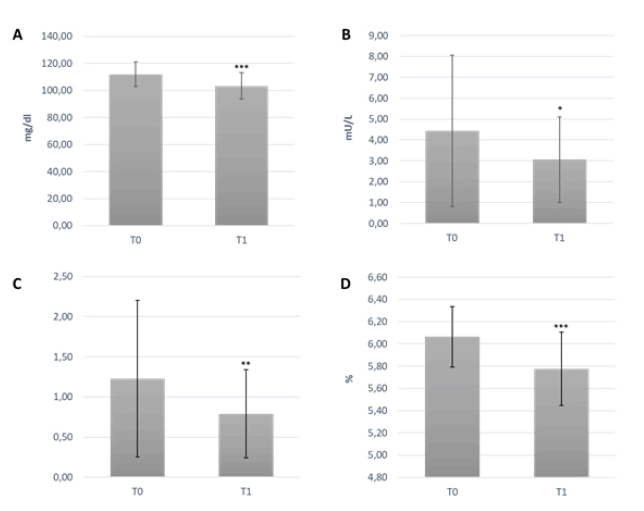
The enrolled population characteristics are summarized in Table 1. Mean age was 62.59 ± 9.31 years, and Body Mass Index (BMI) 28.84 ± 4.23 kg/m2. One subject was excluded due to the occurrence of pregnancy. None experienced adverse events during the trial. After 3 months of supplementation, fasting blood glucose and HbA1c significantly decreased (-7.7% and -4.8%, respectively), when compared to baseline (p<0.0001) (Figure 1). Fasting insulin and HOMA-index were reduced by 31% (p<0.05) and 35.7%, respectively, compared to baseline (p=0.01). There were no significant changes in TC, LDL-c, non-HDL-c and TG levels between T0 and T1. After 3 months, HDL-c increased significantly by 10% when compared to the baseline levels (p<0.05). After 3 months of supplementation, body weight, BMI and abdominal circumference were slightly reduced, however data was non-significant. Blood pressure did not change throughout the study. Final values and differences of all variables (i.e., difference between baseline value and final value) are summarized in Table 2.
| Table 1: Baseline characteristics of the study group. | |
|---|---|
| Study group | 39 subjects |
| Age (years) | 62.59 ± 9.31 |
| Gender (M/F) | 18/21 |
| BMI (Kg/m2) | 28.87 ± 4.28 |
| Hypertension | 41% |
Figure 1: (A) Fasting blood glucose; (B) Fasting insulin; (C) HOMA-index; (D) HbA1c; measures on 39 subjects at the baseline and after 3 months of supplementation with the food supplement based on Mediterranean citrus extracts. One-way repeated measures ANOVA test. *p<0.05, **p ≤ 0.01, ***p ≤ 0.0001 T1 vs baseline.
| Table 2: The variables measured on 39 subjects at the baseline and after 3 months of supplementation with the food supplement. One-way repeated measures ANOVA test. P-value T1 vs baseline. | ||||
|---|---|---|---|---|
| Variable | T0 | T1 | VAR% | p-value |
| Weight (Kg) | 80.61 ± 14.47 | 77.63 ± 13.82 | -3.69 | NS |
| BMI (Kg/m2) | 28.87 ± 4.28 | 27.81 ± 4.09 | -3.69 | NS |
| Total cholesterol (mg/dl) | 191.38 ± 33.37 | 184.77 ± 26.72 | -3.46 | NS |
| HDL (mg/dl) | 52.25 ± 11.06 | 57.54 ± 9.51 | 10.11 | 0.027 |
| non-HDL (mg/dl) | 139.13 ± 32.37 | 127.23 ± 27.38 | -8.55 | NS |
| Triglycerides (mg/dl) | 127.82 ± 40.82 | 113.46 ± 35.75 | -11.23 | NS |
| LDL (mg/dl) | 113.56 ± 32.82 | 104.54 ± 27.23 | -7.95 | NS |
| Systolic pressure | 123.48 ± 8.16 | 121.25 ± 6.93 | -1.81 | NS |
| Diastolic pressure | 72.94 ± 7.50 | 70.92 ± 7.85 | -2.78 | NS |
| Abdominal Circumference (cm) | 104.44 ± 10.99 | 101.61 ± 9.79 | -2.71 | NS |
Discussion
In this study our real life experience on the effects of a nutraceutical compound based on Mediterranean citrus extracts and chromium is reported. The small size of the sample and the lack of a control group characterize the study as a pilot one; the results must be considered as preliminary and require to be confirmed.
The selected patients were adults (more than 40 years); they had a confirmed diagnosis of dysglycemia from almost 2 years, and were in active follow up; according to current guidelines, diagnosis was not confirmed with first line tests (oral glucose tolerance test particularly). The findings of this pilot study show that the daily intake of a nutraceutical compound based on Mediterranean citrus extracts and chromium significantly decreased fasting blood glucose, fasting insulin, HOMA-index and HbA1c levels in dysglycemic outpatients in the short term. HDL-c significantly increased, while TC, LDL-c, non-HDL-c and TG levels decreased, although not significantly. As a healthy nutrition remains the cornerstone of dysglycemia and type 2 diabetes prevention, during the last decades a large attention has been given to flavonoid rich plant extracts[18]. Indeed, their multiple positive health effects on metabolic disorders, diabetes, cardiovascular disease, and obesity have been investigated[17]. Flavonoids exert their antidiabetic activity supporting the regulation of carbohydrate digestion, insulin signalling, insulin secretion, glucose uptake, glucose metabolism, and fat storage[19,20]. Fruits and vegetables are rich in flavonoids, and differences in quantities and type of compounds can be found in juices and peels. Citrus fruits, such as oranges and lemons, contain many flavanones, in particular hesperidin, narirutin, eriocitrin, naringenin. Anthocyanins, belonging to the flavonoids group, are soluble vacuolar pigments that confer the singular red colour to blood oranges (or other types of fruits and vegetables such as red grapes, pomegranate, berries). In particular, oranges are considered a rich source of hesperidin and naringin, the first representing more than 90% of the flavonoids in sweet oranges, and, far behind naringin; red orange juice is rich in anthocyanins and reduces weight and blood lipids in female obese subjects[21-23]. Citrus fruits peel contains the highest concentrations of hesperidin, compared to citrus/orange juices[24]. As a nutraceutical compound it can be considered safe due to its non-accumulative nature[25]. Hesperidin has been shown to play a relevant role in glucose metabolism, both in vitro and in animal models. Indeed, in hepatocyte cell line HepG2, neohesperidin (derived from hesperidin) increased the phosphorylation levels of Adenosine Monophosphate (AMP), Activated Protein Kinase (AMPK) resulting in glucose consumption[26]. Two different studies confirmed the action of hesperidin on the induction of glucose uptake[27,28]. One study showed how hesperidin is able to improve insulin resistance by regulating the insulin receptor substrate 1 (IRS1)-Glucose Transporter (GLUT)-2 pathway via Toll-Like Receptor (TLR)-4. The other investigated also the antioxidant potential of hesperidin and hesperetin under oxidative stress as this is associated with diabetes. Treatment with hesperidin and naringin in diabetic animals reduced the levels of glucose and A1c, as well as inflammatory parameters, such as Tumor Necrosis Factor Alpha (TNFα), Interleukin (IL)-6 and Nitric Oxide (NO)[29]. Thus, these experimental findings demonstrate how hesperidin and naringin exhibit antidiabetic effects against diabetic animal models, by potentiating the antioxidant defence system and inhibiting proinflammatory cytokine production. Hesperidin has been clinically tested in subjects with metabolic syndrome showing a reduction of fasting blood glucose levels after 12 weeks of consumption[30]. Clinical studies have shown a reduction of blood glucose and insulin fasting levels, as well as HOMA index after daily consumption of orange juice in healthy women and obese subjects following a low-calorie diet [31,32]. Naringenin, a saturated oxidized aglycone, has a wide range of biological activities, such as antioxidant, antidiabetic, anti-obesity, anti-inflammatory, and it increases muscle cell glucose uptake via AMPK[33,34]. Eriocitrin has shown to reduce TNF-α, endothelial nitric oxide synthase, intercellular adhesion molecule 1 (ICAM-1), and Vascular Endothelial Growth Factor (VEGF) resulting in lower oxidative stress and inflammation; all these effects are related to improved metabolism of lipids and glucose in animal model[35,36]. Also, anthocyanins have been shown to reduce blood glucose levels by improving insulin sensitivity and insulin secretion and lowering the digestion of sugars within the intestine[37]. Indeed, it has been clinically confirmed that a dietary intake of anthocyanins reduces the risk to develop type II diabetes[38]. Our tested food supplement also contains chromium, a micronutrient involved in different metabolic pathways, that has been shown to regulate blood sugar and reduce insulin resistance [39,40]. The increased cellular glucose uptake exerted by chromium has been explained through its different molecular mechanisms: By the induction of the kinase activity of insulin receptor β, the increased activity of downstream effectors of insulin signaling pI3-kinase and Akt and the Sepmented Glut4 translocation to the cell surface.
Overall, this study shows an improvement of the glucose metabolic parameters in subjects with prediabetes after three months of supplementation with the Mediterranean citrus complex. A positive impact was seen also on the lipid profile, although not significant; only HDL was significantly improved. A slight not significant body weight and waist circumference decrease has been observed: it may be due to a higher adherence during the new approach to the care (“trial effect”). Indeed, our results are in line with other clinical studies that reported an inconclusive effect of hesperidin on CVD risk factors, such the lipid factors[41]. Therefore, the synergy between citrus extracts rich in flavonoids, such as hesperidin and anthocyanins, in combination with chromium could be an effective and safe option to regulate the plasma glucose levels in dysglycemic subjects.
Conclusion
Strength of this pilot study is the first demonstration of an in vivo effect of a nutraceutical compound, containing a flavonoid complex from citrus, in regulating the plasma glucose levels in dysglycemic subjects. The real life setting excluded significant side effects and put in evidence a good tolerance and satisfaction of the patients who had a good adherence to the therapy.
Limitations are the small number of included subjects and the lack of a control group. Further long-term controlled studies are warranted to confirm the effect of citrus flavonoids in combination with chromium in the management of dysglycemia.
References
- Hostalek U. Global epidemiology of prediabetes-present and future perspectives. Clin Diabetes Endocrinol. 2019;5:5.
[Crossref], [Google Scholar], [Indexed]
- https://aemmedi.it/wp-content/uploads/2009/06/AMD-Standard-unico1.pdf
- The Task Force on diabetes, Pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC), European Association for the Study of Diabetes (EASD), Rydén L, Grant PJ, Stefan DA, et al. ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD – Summary. Diab Vasc Dis Res. 2014;11:133–73.
[Crossref], [Google Scholar], [Indexed]
- Fonseca VA. Defining and characterizing the progression of type 2 diabetes. Diabetes Care. 2009;32:S151–6.
[Crossref], [Google Scholar], [Indexed]
- Brannick B, Dagogo-Jack S. Prediabetes and cardiovascular disease: Pathophysiology and interventions for prevention and risk reduction. Endocrinol Metab Clin North Am. 2018;47:33–50.
[Crossref], [Google Scholar], [Indexed]
- Rett K, Gottwald-Hostalek U. Understanding prediabetes: Definition, prevalence, burden and treatment options for an emerging disease. Curr Med Res Opin. 2019;35:1529–34.
[Crossref], [Google Scholar], [Indexed]
- Tabák AG, Herder C, Rathmann W, Brunner EJ, Kivimäki M. Prediabetes: A high-risk state for diabetes development. The Lancet. 2012;379:2279–90.
[Crossref], [Google Scholar], [Indexed]
- Zand A, Ibrahim K, Patham B. Prediabetes: Why Should We Care?. Methodist DeBakey Cardiovasc J. 2018;14:289.
[Crossref], [Google Scholar], [Indexed]
- Huang Y, Cai X, Mai W, Li M, Hu Y. Association between prediabetes and risk of cardiovascular disease and all cause mortality: Systematic review and meta-analysis. BMJ. 2016 Nov 23;i5953.
[Crossref], [Google Scholar], [Indexed]
- Stratton IM. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): Prospective observational study. BMJ. 2000;32:405–12.
[Crossref], [Google Scholar], [Indexed]
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). The lancet.1998;352:837-853.
[Crossref], [Google Scholar], [Indexed]
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1204764/
- https://europepmc.org/article/med/17802973
- Ramanathan amnath S. Correlation of duration, hypertension and glycemic control with microvascular complications of diabetes mellitus at a tertiary care hospital. Integr Mol Med. 2022;1.
[Crossref], [Google Scholar]
- Knowler. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. [Crossref], [Google Scholar], [Indexed]
- Herman WH, Hoerger TJ, Brandle M, Hicks K, Sorensen S, Zhang P, et al. The cost-effectiveness of lifestyle modification or metformin in preventing type 2 diabetes in adults with impaired glucose tolerance. Ann Intern Med. 2005;142:323.
[Crossref], [Google Scholar], [Indexed]
- Giacco R, Costabile G, Fatati G, Frittitta L, Maiorino MI, Marelli G, et al. Effects of polyphenols on cardio-metabolic risk factors and risk of type 2 diabetes. A joint position statement of the Diabetes and Nutrition Study Group of the Italian Society of Diabetology (SID), the Italian Association of Dietetics and Clinical Nutrition (ADI) and the Italian Association of Medical Diabetologists (AMD). Nutr Metab Cardiovasc Dis. 2020;30:355–67.
[Crossref], [Google Scholar], [Indexed]
- Schulze MB, Hu FB. Primary prevention of diabetes: What can be done and how much can be prevented?. Annu Rev Public Health. 2005;26:445–67.
[Crossref], [Google Scholar], [Indexed]
- AL-Ishaq, Abotaleb, Kubatka, Kajo, Büsselberg. Flavonoids and their anti-diabetic effects: Cellular mechanisms and effects to improve blood sugar levels. Biomolecules. 2019;9:430.
[Crossref], [Google Scholar], [Indexed]
- Vinayagam R, Xu B. Antidiabetic properties of dietary flavonoids: A cellular mechanism review. Nutr Metab. 2015;12:60.
[Crossref], [Google Scholar], [Indexed]
- Erlund I. Review of the flavonoids quercetin, hesperetin, and naringenin. Dietary sources, bioactivities, bioavailability, and epidemiology. Nutr Res. 2004;24:851–74.
[Crossref], [Google Scholar]
- Khan MK, Zill-E-Huma, Dangles O. A comprehensive review on flavanones, the major citrus polyphenols. J Food Compos Anal. 2014;33:85–104.
[Crossref], [Google Scholar]
- Azzini E, Venneria E, Ciarapica D, Foddai MS, Intorre F, Zaccaria M, et al. Effect of red orange juice consumption on body composition and nutritional status in overweight/obese female: A pilot study. Oxid Med Cell Longev. 2017;2017:1–9.
[Crossref], [Google Scholar], [Indexed]
- Tomàs-Barberàn FA, Clifford MN. Flavanones, chalcones and dihydrochalcones - nature, occurrence and dietary burden. J Sci Food Agric. 2000;80:1073–80.
[Crossref], [Google Scholar]
- Hajialyani M, Hosein Farzaei M, Echeverría J, Nabavi S, Uriarte E, Sobarzo-Sánchez E. Hesperidin as a neuroprotective agent: A review of animal and clinical evidence. Molecules. 2019;24:648.
[Crossref], [Google Scholar], [Indexed]
- Zhang J, Sun C, Yan Y, Chen Q, Luo F, Zhu X, et al. Purification of naringin and neohesperidin from Huyou (Citrus changshanensis) fruit and their effects on glucose consumption in human HepG2 cells. Food Chem. 2012;135:1471–8.
[Crossref], [Google Scholar], [Indexed]
- Xuguang H, Aofei T, Tao L, Longyan Z, Weijian B, Jiao G. Hesperidin ameliorates insulin resistance by regulating the IRS1‐GLUT2 pathway via TLR4 in HepG2 cells. Phytother Res. 2019;33:1697–705.
- Dhanya R, Jayamurthy P. In vitro evaluation of antidiabetic potential of hesperidin and its aglycone hesperetin under oxidative stress in skeletal muscle cell line. Cell Biochem Funct. 2020;38:419–27.
[Crossref], [Google Scholar], [Indexed]
- Mahmoud AM, Ashour MB, Abdel-Moneim A, Ahmed OM. Hesperidin and naringin attenuate hyperglycemia-mediated oxidative stress and proinflammatory cytokine production in high fat fed/streptozotocin-induced type 2 diabetic rats. J Diabetes Complications. 2012;26:483–90.
[Crossref], [Google Scholar], [Indexed]
- Yari Z, Movahedian M, Imani H, Alavian SM, Hedayati M, Hekmatdoost A. The effect of hesperidin supplementation on metabolic profiles in patients with metabolic syndrome: A randomized, double-blind, placebo-controlled clinical trial. Eur J Nutr. 2020;59:2569–77.
[Crossref], [Google Scholar], [Indexed]
- Lima ACD, Cecatti C, Fidélix MP, Adorno MAT, Sakamoto IK, Cesar TB, et al. Effect of daily consumption of orange juice on the levels of blood glucose, lipids, and gut microbiota metabolites: Controlled clinical trials. J Med Food. 2019;22:202–10.
[Crossref], [Google Scholar], [Indexed]
- Ribeiro C, Dourado G, Cesar T. Orange juice allied to a reduced-calorie diet results in weight loss and ameliorates obesity-related biomarkers: A randomized controlled trial. Nutrition. 2017;38:13–9.
[Crossref], [Google Scholar], [Indexed]
- Patel K, Singh GK, Patel DK. A review on pharmacological and analytical aspects of naringenin. Chin J Integr Med. 2018;24:551–60.
[Crossref], [Google Scholar], [Indexed]
- Zygmunt K, Faubert B, MacNeil J, Tsiani E. Naringenin, a citrus flavonoid, increases muscle cell glucose uptake via AMPK. Biochem Biophys Res Commun. 2010;398:178–83.
[Crossref], [Google Scholar], [Indexed]
- Ferreira PS, Manthey JA, Nery MS, Spolidorio LC, Cesar TB. Low doses of eriocitrin attenuate metabolic impairment of glucose and lipids in ongoing obesogenic diet in mice. J Nutr Sci. 2020;9:e59.
[Crossref], [Google Scholar], [Indexed]
- Ferreira PS, Spolidorio LC, Manthey JA, Cesar TB. Citrus flavanones prevent systemic inflammation and ameliorate oxidative stress in C57BL/6J mice fed high-fat diet. Food Funct. 2016;7:2675–81.
[Crossref], [Google Scholar], [Indexed]
- Mukherjee PK. Phyto-pharmaceuticals, nutraceuticals and their evaluation. In: Quality control and evaluation of herbal drugs. Elsevier; 2019;707–22.
- Wedick NM, Pan A, Cassidy A, Rimm EB, Sampson L, Rosner B, et al. Dietary flavonoid intakes and risk of type 2 diabetes in US men and women. Am J Clin Nutr. 2012;95:925–33.
[Crossref], [Google Scholar], [Indexed]
- Balk EM, Tatsioni A, Lichtenstein AH, Lau J, Pittas AG. Effect of chromium supplementation on glucose metabolism and lipids. Diabetes Care. 2007;30:2154–63.
[Crossref], [Google Scholar], [Indexed]
- Hua Y, Clark S, Ren J, Sreejayan N. Molecular mechanisms of chromium in alleviating insulin resistance. J Nutr Biochem. 2012;23:313–9.
[Crossref], [Google Scholar], [Indexed]
- Mas-Capdevila A, Teichenne J, Domenech-Coca C, Caimari A, Del Bas JM, Escoté X, et al. Effect of hesperidin on cardiovascular disease risk factors: The role of intestinal microbiota on hesperidin bioavailability. Nutrients. 2020;12:1488.
[Crossref], [Google Scholar], [Indexed]




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.