Osteogenesis Imperfecta: A Case Report and Review of Literature
- *Corresponding Author:
- Dr. Edelu BO
Department of Paediatrics, University of Nigeria Teaching Hospital, Enugu, Nigeria.
E-mail: onyedelu@yahoo.com
Abstract
Osteogenesis imperfecta (OI) is a group of rare inherited disorders of connective tissue with the common feature of excessive fragility of bones. It is caused by mutations in the collagen, type I, alpha 1 and collagen type I alpha 2 genes, which encode the alpha 1 and the alpha 2 chain of type I procollagen, respectively. Molecular genetic studies have detected more than 150 mutations of these genes. Other mutations have been identified in the leucine proline‑enriched proteoglycan 1, cartilage associated protein and peptidyl‑proly lisomerase B genes.
Keywords
Brittle bone disease and fragile bone disease, Fragilitas ossium, Osteogenesis imperfecta, Osteopetrosis idiopathica
Introduction
Osteogenesis imperfecta (OI) is a group of rare inherited disorders of connective tissue with the common feature of excessive fragility of bones.[1] It is caused by mutations in the collagen, type I, alpha 1 and collagen type I alpha 2 genes, which encode the alpha 1 and the alpha 2 chain of type I procollagen, respectively.[2] Molecular genetic studies have detected more than 150 mutations of these genes.[2] Other mutations have been identified in the leucine proline-enriched proteoglycan 1, cartilage associated protein and peptidyl-proly lisomerase B genes.[3,4]
The incidence is 1:20,000 and occurs in all races and ethnicity.[5] It is mostly inherited as an autosomal dominant disorder, but autosomal recessive forms have been reported.[6,7] The clinical classification by Sillence et al., is the most helpful in prognosis and genetic counseling and it groups OI into four types: Type I OI is mild, type II is perinatal lethal, type III is progressive deforming and type IV is moderately severe.[8] It is characterized by multiple and recurrent fractures, which are intrauterine or perinatal or post-natal. Other features include blue sclera, otosclerosis with hearing loss, high arched palate, hyperlaxity of ligaments and skin, “dentinogenesis imperfecta” (defective dentition), scoliosis and growth retardation. Wormian bones could also be seen on skull X-ray. Intelligence is not affected.[3]
Diagnosis can be made clinically. Radiographic support and confirmation by collagen analysis of skin fibroblast culture or blood deoxyribonucleic acid analysis may be necessary in some cases.[5,9] This is important for genetic counseling and cases of suspected child abuse. Prenatal diagnosis for at risk pregnancies by fetal ultrasonography in the early 2nd trimester is possible and enables care.[5,10] There’s no cure for OI. Management is multidisciplinary involving mainly surgery, physiotherapy and rehabilitation. However, medical treatment, especially with bisphosphonate have shown good prospects.[3,5,9]
This case is being reported because of its rarity, especially among blacks and also, to review the literature to highlight the challenges and advances in its management.
Case Report
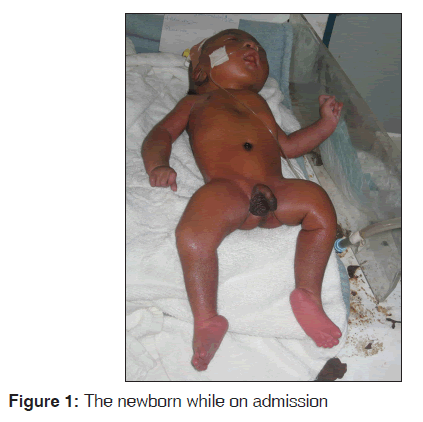
O.E [Figure 1] was a male neonate delivered to a 33-year-old para 2 female at University of Nigeria Teaching Hospital, Enugu. He was admitted to the new born special care unit (NBSCU) 10 min after birth for inability to cry at birth, multiple deformities with abnormal body posture.
His mother received routine antenatal care and pregnancy was uneventful. Only routine drugs were used during pregnancy. Prenatal ultrasonography detected no fractures or other abnormalities. Onset of labor was spontaneous and lasted about 8 h with spontaneous vertex delivery. Apgar score was four at 10 min. There was no history of the tendency to fractures in the family and no family history of any baby with fracture at birth.
On examination in the NBSCU, he was found to be in “frog like position” with abnormal movement of the left thigh. He was in respiratory distress with acrocyanosis. He had blue-grey sclera and micrognathia with a high arched palate. Musculoskeletal system examination revealed pectus excavatum, fixed flexion deformity of the fingers, crepitus of the right humerus and ulnar and both femurs with bilateral talipes equinovarus and micromelia.
A diagnosis of severe birth asphyxia in a neonate with OI type II was made. Radiograph revealed fractures of the right humerus, ulna and femur with osteopenia. Treatment was mainly supportive with minimal handling, intermittent suctioning and intra-nasal oxygen administration. The parents were counseled and the baby was also reviewed by the orthopaedic surgeons and anesthetist for later surgical intervention when he stabilized. However, he continued to deteriorate until his death on the 7th day of life.
Discussion/Literature Review
OI is a rare disorder. It has variously been called “osteopsathyrosis idiopathica” by Lobstein, “fragilitus osseum”, hereditary fragility of bone, later brittle bone disease and fragile bone disease, but the current name “OI” was coined by Vrolik in 1845.[11,12] The disorder was first brought to the limelight by the Swede, Olof Jakob Ekman in his doctorate thesis at Uppsala in 1788 as “osteomalacia congenital” involving three generations.[12,13] Since then, various researchers have tried to describe and classify this disorder into various types: In 1906, Looser, proposed the classification: “Congenita” and “tarda” based on time of fractures.[14] Congenita type has fracture in-utero and most were either still birth or died soon after. OI congenita was found in children whose parents have no traces of the disease while OI tarda was the hereditary form. In 1975, Bauze et al.,[15] describing the clinical, radiological and biochemical features of 42 patients classified OI into three: mild, moderate and severe based on deformity of one or more of the long bones. Mild had no long bone deformity; moderate had minor long bone deformities while severe had severe long bone deformities. One of their arguments is that long bone deformity is a strong prognostic feature and easily recognizable.
The most widely acceptable classification was by Sillence et al.,[8] in 1979, in which they grouped the disorder into four: Type I; dominantly inherited OI with blue sclera, type II; perinatally lethally deforming OI with crumpled femora, type III; progressively deforming OI with normal sclera and type IV; dominantly inherited OI with normal sclerae. Type I is a mild form with recurrent fractures and blue sclera. It has A and B subtypes depending on the absence or presence of dentinogenesis imperfecta. Fractures are less common after puberty. Type II is autosomal recessive and generally lethal in the newborn period with multiple congenital fractures, micromelia and severe lung disease. Types III and IV represent intermediate phenotypes with type III being the most severe non-lethal form causing significant bony deformity secondary to multiple fractures, which can be congenital. Type IV also has subtypes A and B based on the presence of dentinogenesis imperfecta.
Over the past few decades, five additional types (V-IX) have been identified using histological and molecular findings.[6,7] Plotkin[16] proposed a classification of OI as syndromes secondary to mutations in type I pro-collagen genes and “syndromes resembling osteogenesis imperfecta” as those secondary to mutations other than the type I pro-collagen genes.
The diagnosis of OI type II in our patient was based on the clinical features of multiple fractures at birth, blue-grey sclera, micromelia and radiological evidence of osteopenia. Although, there was no positive family history suggestive of OI or other skeletal malformations, it was noted that sporadic cases were most likely in type II.[8] Akiode et al., in Sagamu,[17] and Akinola et al., in Lagos,[18] both in Western Nigeria reported a single and two cases respectively of female babies based on the clinical findings of multiple fractures, blue sclera and craniotabes at birth with radiological evidence of defective ossification of the skull.
Although, reports available,[5,19] show no racial or ethnic variation, the sparseness of reports from Africa and obvious absence of blacks in most of the case reports from other continents may be an indication of low incidence in Africans. It may also be as a result of under reporting due to wide unavailability of facilities for disease confirmation. Although, an autosomal recessive form of type III, which is very rare, has been reported as been relatively more common among South African black population,[20] the etiology is not certain as type I collagen structural genes Colia one and two have not been demonstrated to be mutated in the affected population.[21] There have also been reports of OI in twins from Burundi[22] and more recently Nigeria.[23]
Prenatal diagnosis by fetal ultrasonography is possible,[5] but no abnormality was detected in our patient prenatally. Likewise, in the first case earlier reported within the country. The other case, report did not state the ultrasonography findings. It’s also possible that the fractures occurred during delivery in both cases since prenatal diagnosis is mainly based on the presence of fractures. Fractures can occur even on minimal stress, but there is no empiric data to prove that caesarean section will improve outcome over vaginal birth even when diagnosed prenatally.[24] However, its poor prognostic course raises an ethical question of termination of pregnancy on detection. Despite this, advances in technology continue to proffer options for these children.
Surgery had been the main stay of management of OI with supportive physiotherapy and rehabilitation. However, the persistent brittleness of the bones with repeated fractures made researchers to seek alternative treatment that will permanently strengthen the bone structure. Medical treatment, though dating back to about two centuries ago, thus started to dominate discussions over the past few decades as an adjunct.
Experiments on medical management started as far back as 1897 with Griffith who combined phosphorus, codliver oil, fresh air and exercise.[25] Since then, over 25 different agents including hormones such as pituitary, thyroid, parathyroid, androgens, estrogens and calcitonin, vitamins A, C and D as well as mineral agents such as strontium, aluminum, fluoride, magnesium and citrate of sodium hydroxide have been used in attempts to treat OI.[26] Just like in recent years, most of the researchers claimed some benefits for their products but none was recorded to be a success.
Almost a century later, Devogelaer et al.,[27] introduced the use of bisphosphonates, which appeared to have brought the hope of a successful medical treatment. Their trial was based on the use of the drug in the treatment of other bone disorders like juvenile osteoporosis and Paget disease of the bone.
Bisphosphonates (diphosphonates) are derived from pyrophosphate, a naturally occurring agent that slows or stops the naturally occurring process of bone resorption, thereby resulting in increased bone density and strength. Bisphosphonates have evolved over time from its original compound to 2nd and 3rd generation aminobisphosphonates such as pamidronate, alendronate and risedronate.
Since this first report in 1987, numerous researchers have tried to evaluate the use of bisphosphonates in the treatment of OI using different regimens, with over a hundred publications and still counting. Cyclic IV pamidronate appears to be the most widely reported treatment for children with OI. Most researchers reported increase in bone mineral density as well as reduction in the risk of fracture.[28-30] There are also a report on increased height and weight in children treated with pamidronate.[31] Risedronate has been proposed as a more suitable alternative to pamidronate because of its lower mineral affinity than the other nitrogen-containing bisphosphonates such as alendronate and olpadronate.[32] Antoniazzi et al.,[33] also demonstrated that a combination of bisphosphonate and growth hormone produced a better result in terms of bone mineral density, lumbar spine projected area and growth velocity than bisphosphonate alone.
The aim of these treatments should be to increase bone strength and thus reduce to the barest minimum the rate of fractures as well as prevent deformities of long bone and spine. This should improve the general well-being of the affected individuals. However, despite these reports by the various researchers, of improvement in bone mineral density, linear growth, reduced rates of fractures and chronic bone pain, the ultimate goal of treatment is yet to be achieved. There are even concerns on how long treatment should be continued to achieve a sustained result, the safety of prolonged use of bisphosphonates on children,[9,34,35] as well as delay in healing of fractures after osteotomy.[36] Rauch et al.,[36] observed very little benefit after 2 to 4 years of therapy. Even the documented weight increase,[31] is a source of concern as it’s likely to affect negatively the rate of fractures and effective physical rehabilitation of the patients.
Bone marrow transplant as a treatment strategy has been evaluated with documented improvement in linear growth, total body bone mineral content and reduction in fracture rate.[37] The principle behind this success stems from the fact that bone marrow mesenchymal cells could differentiate into a variety of tissues including bone and cartilage. The increased level of osteoblasts resulting from the transplant ultimately increases collagen formation. Although researchers[12] had earlier pointed out that the problem in OI is not a decrease in bone formation, rather an increase in bone resorption, it may be argued that the increased formation of osteoblasts are able to match the rate of resorption, resulting in the effects obtained. The question, like in the use of bisphosphonates, is the issue of long-term benefit.
There is no doubt that medical treatment has brought about some respite for children with OI, ultimately, a cure for OI is expected to stem from a re-engineering or elimination of the mutated gene. The Gene therapy approach is being investigated as potential for future treatments of OI.[38] Until then, palliative treatment remains the only option.
Conclusion
OI is a rare inherited disorder, especially among blacks. This case has been reported as one of the few cases coming from Africa. This report from Nigeria and other reports from other parts of Africa call for more alertness to identify the case of babies who may have OI. The review of literature highlights the challenges in the latest progress in the management. There is still need for further research toward improved morbidity of the disease and possibly a cure.
Source of Support
Nil.
Conflict of Interest
None declared.
References
- Smith R. Disorders of the skeleton. In: Weatherall DJ,Ledingham JG, Warrell DA, editors. Oxford Textbook of Medicine. Oxford: Oxford University Press; 1984. p. 17.25.
- Burton BK, Charrow J. Other important single gene disorders. In: Green TP, Franklin WH, Tanz RR, editors. Pediatrics: Just the Facts. Boston: McGraw Hill; 2005. p. 3467.
- Polousky JD, Eilert RE. Orthopedics. In: Hay WW, Levin MJ, Sondheimer JM, Deterding RR, editors. Current Pediatrics: Diagnosis and Treatment. Vol. 753, 19th ed. New York: McGraw Hill; 2009. p. 10056.
- Cabral WA, Chang W, Barnes AM, Weis M, Scott MA, Leikin S, et al. Prolyl 3hydroxylase 1 deficiency causes a recessive metabolic bone disorder resembling lethal/severe osteogenesis imperfecta. Nat Genet 2007;39:35965.
- Marini JC. Osteogenesis imperfecta. In: Nelson WE, Behrman RE, Kliegman RM, Arvin AM, editors. Nelson Textbook of Pediatrics. 18th ed. Philadelphia: W.B. Saunders Company; 2007. p. 288790.
- Ward LM, Rauch F, Travers R, Chabot G, Azouz EM, Lalic L, et al. Osteogenesis imperfecta type VII: An autosomal recessive form of brittle bone disease. Bone 2002;31:128.
- Glorieux FH, Rauch F, Plotkin H, Ward L, Travers R, Roughley P, et al. Type V osteogenesis imperfecta: A new form of brittle bone disease. J Bone Miner Res 2000;15:16508.
- Sillence DO, Senn A, Danks DM. Genetic heterogeneity in osteogenesis imperfecta. J Med Genet 1979;16:10116.
- Rauch F, Glorieux FH. Osteogenesis imperfecta. Lancet 2004;363:137785.
- Stephens JD, Filly RA, Callen PW, Golbus MS. Prenatal diagnosis of osteogenesis imperfecta type II by realtime ultrasound. Hum Genet 1983;64:1913.
- Brailsford JF. Osteogenesis imperfecta. Br J Radiol 1943;16:12936.
- Robichon J, Germain JP. Pathogenesis of osteogenesis imperfecta. Can Med Assoc J 1968;99:9759.
- Carey MC, Fitzgerald O, McKiernan E. Osteogenesis imperfecta in twentythree members of a kindred with heritable features contributed by a nonspecific skeletal disorder. Q J Med 1968;37:43749.
- Chawla S. Intrauterine osteogenesis imperfecta in four siblings. Br Med J 1964;1:99101.
- Bauze RJ, Smith R, Francis MJ. A new look at osteogenesis imperfecta. A clinical, radiological and biochemical study of fortytwo patients. J Bone Joint Surg Br 1975;57:212.
- Plotkin H. Syndromes with congenital brittle bones. BMC Pediatr 2004;4:16.
- Akiode O, Ogunfowora OB, Shonubi A, Bajoma AA, Musa AA, Sotimehin SA. Osteogenesis imperfecta in a Nigerian baby. SICOT Online Report E049; 2003. [Last cited on 2012 Aug].
- Akinola R, Disu E, Adewale O. Osteogenesis imperfecta: A report of two cases. Internet J Pediatr Neonatol 2008;8(2).
- Byers PH, Steiner RD. Osteogenesis imperfecta. Annu Rev Med 1992;43:26982.
- Beighton P, Versfeld GA. On the paradoxically high relative prevalence of osteogenesis imperfecta type III in the black population of South Africa. Clin Genet 1985;27:398401.
- Wallis GA, Sykes B, Byers PH, Mathew CG, Viljoen D, Beighton P. Osteogenesis imperfecta type III: Mutations in the type I collagen structural genes, COL1A1 and COL1A2, are not necessarily responsible. J Med Genet 1993;30:4926.
- Armstrong O, Karayuba R, Ngendahayo L, Habonimana E. Osteogenesis imperfecta in monozygotic twins in Burundi. Med Trop (Mars) 1994;54:5962.
- Fajolu IB, Ezeaka VC, Elumelu OJ, Onabajo OA, Ananti CH, Iroha EO et al. Osteogenesis Imperfecta in a set of Nigerian twinsA case report. Internet J Pediatr Neonatol 2012;(14) doi:10.5580/2c29.
- Cubert R, Cheng EY, Mack S, Pepin MG, Byers PH. Osteogenesis imperfecta: Mode of delivery and neonatal outcome. Obstet Gynecol 2001;97:669.
- Albright JA. Systemic treatment of osteogenesis imperfecta. Clin Orthop Relat Res 1981;:8896.
- Albright JA. Systemic treatment of osteogenesis imperfectaHistoric review. Abstract of symposium II on osteogenesis imperfecta. Curr Orthop Pract 1976;116:258.
- Devogelaer JP, Malghem J, Maldague B, Nagant de Deuxchaisnes C. Radiological manifestations of bisphosphonate treatment with APD in a child suffering from osteogenesis imperfecta. Skeletal Radiol 1987;16:3603.
- Plotkin H, Rauch F, Bishop NJ, Montpetit K, RuckGibis J, Travers R, et al. Pamidronate treatment of severe osteogenesis imperfecta in children under 3 years of age. J Clin Endocrinol Metab 2000;85:184650.
- Glorieux FH, Bishop NJ, Plotkin H, Chabot G, Lanoue G, Travers R. Cyclic administration of pamidronate in children with severe osteogenesis imperfecta. N Engl J Med 1998;339:94752.
- Falk MJ, Heeger S, Lynch KA, DeCaro KR, Bohach D, Gibson KS, et al. Intravenous bisphosphonate therapy in children with osteogenesis imperfecta. Pediatrics 2003;111:5738.
- Zeitlin L, Rauch F, Plotkin H, Glorieux FH. Height and weight development during four years of therapy with cyclical intravenous pamidronate in children and adolescents with osteogenesis imperfecta types I, III, and IV. Pediatrics 2003;111:10306.
- Bishop N, Harrison R, Ahmed F, Shaw N, Eastell R, Campbell M, et al. A randomized, controlled doseranging study of risedronate in children with moderate and severe osteogenesis imperfecta. J Bone Miner Res 2010;25:3240.
- Antoniazzi F, Monti E, Venturi G, Franceschi R, Doro F, Gatti D, et al. GH in combination with bisphosphonate treatment in osteogenesis imperfecta. Eur J Endocrinol 2010;163:47987.
- Castillo H, SamsonFang L, American Academy for Cerebral Palsy and Developmental Medicine Treatment Outcomes Committee Review Panel. Effects of bisphosphonates in children with osteogenesis imperfecta: An AACPDM systematic review. Dev Med Child Neurol 2009;51:1729.
- Marini JC. Do bisphosphonates make children’s bones better or brittle? N Engl J Med 2003;349:4236.
- Rauch F, Travers R, Glorieux FH. Pamidronate in children with osteogenesis imperfecta: Histomorphometric effects of longterm therapy. J Clin Endocrinol Metab 2006;91:5116.
- Horwitz EM, Prockop DJ, Gordon PL, Koo WW, Fitzpatrick LA, Neel MD, et al. Clinical responses to bone marrow transplantation in children with severe osteogenesis imperfecta. Blood 2001;97:122731.
- Niyibizi C, Wang S, Mi Z, Robbins PD. Gene therapy approaches for osteogenesis imperfecta. Gene Ther 2004;11:40816.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.