Oxidative Stress ‑a Phenotypic Hallmark of Fanconi Anemia and Down Syndrome: The Effect of Antioxidants
- *Corresponding Author:
- Dr. Hala T El Bassyouni
Department of Clinical Genetics, National Research Centre, El-Tahreer street, Dokki, Cairo, Egypt.
E-mail: halabassyouni@yahoo.com
Abstract
Background: Oxidative stress plays a major role in the pathogenesis of leukemia‑prone diseases such as Fanconi anemia (FA) and Down syndrome (DS). Aim: To explore the oxidative stress state in children with DS and FA by estimating the levels of antioxidants (e.g., malondialdehyde [MDA], total antioxidant capacity, and superoxide dismutase [SOD] activity) and DNA damage, and to evaluate of the effect of antioxidant treatment on these patients. Subjects and Methods: The study included 32 children clinically diagnosed with (15 patients) and FA (17 patients) in addition to 17 controls matched for age and sex. MDA, total antioxidant capacity, SOD activity, and DNA damage were measured. Antioxidants including Vitamin A, E, and C were given to the patients according to the recommended daily allowance for 6 months. Clinical follow‑up and re‑evaluation were conducted for all patients. Laboratory tests including complete blood count, karyotyping, DNA damage, and oxidative stress were re‑evaluated. Statistical analysis was performed using statistical computer program Statistical Package for the Social Sciences version 14.0. Results: Children with FA and DS had elevated levels of oxidative stress and more DNA damage than controls. Oxidative stress parameters and DNA damage improved in FA and DS patients after antioxidant administration. Conclusion: Early administration of antioxidants to FA and DS patients is recommended for slowing of the disease course with symptoms amelioration and improvement of general health.
Keywords
Down syndrome, Oxidative DNA damage, Oxidative stress
Introduction
In Fanconi anemia (FA) and Down syndrome (DS), chromosomal aberrations [1] and cancer proneness are hallmark features shared by these two disorders, as well as other manifestations including early aging, immunological dysfunction, and congenital malformations.[2]
Fanconi anemia (MIM 227650) is a rare autosomal recessive disorder featuring bone marrow failure, proneness to malignancies, and chromosomal instability.[3,4] FA is diagnosed by detection of excess chromosomal instability induced by diepoxybutane (DEB) or mitomycin C.[5,6] The high frequency of chromosomal breaks in FA has been related to the increased oxidative damage via the intermediacy of superoxide that stimulates further superoxide production. This long-lasting process may exceed the DNA repair system leading to progressive impairment of bone marrow and predisposing patients to leukemia.[7]
Down syndrome (MIM 190685) is caused by trisomy 21 and the over-expression of the genes present on the extra chromosome 21. DS is the most common aneuploidy, occurring as frequently as approximately one in every 700 live births.[8,9] The evidence of increased oxidative stress in DS is strong and includes gene dose overexpression of superoxide dismutase (SOD), increased lipid peroxidation, and increased oxidative DNA damage. Oxidative stress may have a direct role in producing the neuropathology of DS.[10]
Genome integrity in FA and DS has diagnostic and prognostic importance because of the evident relationship between genome instability and both DNA damage and cancer predisposition.[11] DNA damage is detected by Comet assay represented by an increase of DNA fragments that have migrated out of the cell nucleus in the form of a characteristic streak similar to the tail of a comet.[12]
Antioxidant administration plays an important role in improving the disease course by decreasing oxidative stress-associated complications. [2,13,14]
The aim of the study was to explore the oxidative stress state in children with DS and FA by estimating the levels of antioxidants (malondialdehyde (MDA), total antioxidant capacity and SOD activity) and DNA damage, and to evaluate the effect of antioxidant treatment on these patients.
Subjects and Methods
Patients enrolled in the study were selected randomly from patients referred to the outpatient clinic of the Clinical Genetics Department, NRC, Egypt and they were followed-up for 1½ years during 2012–2014. The patients’ ages ranged between 2 and 10 years. The study included 32 children clinically diagnosed as FA (group I: 17 patients), DS (group II: 15 patients) in addition to 17 controls (group III) matched for age and sex. An informed consent was signed by the parents. The study was approved by the Ethical Committee of the National Research Centre.
Exclusion criteria
• Children (patients or controls) taking antioxidants or multivitamins containing antioxidants
• Transplanted FA patients.
All children enrolled in the study were subjected to:
I. Clinically:
• Full history taking
• Pedigree construction
• Thorough clinical examination of all body systems to detect any system abnormality
• Essential anthropometric measures [15]
II. Laboratory investigations at the start of the study:
• Complete blood count (CBC) for FA patients
• Cytogenetic study:
• Conventional cytogenetic study [16]
• Induction of chromosomal breakage by the DEB for FA patients to confirm the diagnosis of FA and to determine the degree of the chromosomal breakage before starting the antioxidant therapy [17,18]
• Comet assay: DNA damage was evaluated in leukocytes [19]
• Estimation of Oxidative stress parameters including:
• Thiobarbituric acid reactive species (MDA) as a marker of lipid peroxidation [20]
• Total antioxidant capacity biomarker [21]
• Superoxide dismutase biomarker as an antioxidant enzyme [22]
III. Antioxidants therapy: Including Vitamin A, E, and C were given to the patients according to the recommended daily allowance for 6 months. Vitamin A was given in a dose of 4000 IU/day, Vitamin E in 10 mg/day, and Vitamin C in 25 mg/day
IV. Clinical follow-up every month: All patients were clinically examined every month to assure their compliance to treatment and check for any complaints
V. Re-evaluation after 6 months of treatment:
• Clinical re-evaluation and essential anthropometric measurements were done for all patients
• Laboratory tests including CBC, evaluation of the DNA damage level was done by DEB test and comet assay and the values of oxidative stress were repeated to evaluate the effect of antioxidant therapy.
Statistical analysis
Statistical analysis was performed on IBM/PC using statistical computer program Statistical Package for the Social Sciences (SPSS) version 14.0 for windows (SPSS Inc. Chicago, USA). The tests used were descriptive test, Pearson’s correlation test (correlation coefficient r) to test a positive or negative linear relationship between two variables and simple t- test (p - values < 0.05 were considered significant).
Results
Demographic and clinical features of FA patients (group I) are summarized in Table 1.
| Parameter | Number | Frequency (%) |
|---|---|---|
| Male/female | 9/8 | 1.8/1 |
| Positive parental consanguinity | 15/17 | 88.2 |
| Similarly affected family members | 10/17 | 58.8 |
| Epistaxis, petechiae, and ecchymosis | 17/17 | 100 |
| Skin hyperpigmentation | 11/17 | 64.7 |
| Short stature | 11/17 | 64.7 |
| Microcephaly | 9/17 | 52.9 |
| Underweight | 6/17 | 35.3 |
| Radial reduction defects | 6/17 | 35.3 |
| Respiratory disease | 6/17 | 35.3 |
| Renal disease | 5/17 | 29.4 |
| Hypopigmented areas | 3/17 | 17.6 |
| Congenital heart defect | 3/17 | 17.6 |
| GIT problems | 2/17 | 14.3 |
| Growth hormone deficiency | 2/17 | 14.3 |
| Eye affection | 1/17 | 5 |
| Gonadal affection | 1/17 | 5 |
| Simian creases | 1/17 | 5 |
Table 1: Clinical data of group I (Fanconi anemia)
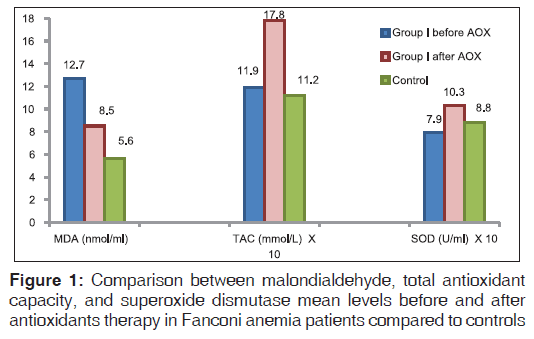
Oxidative stress parameters in FA patients revealed significantly higher MDA levels (P < 0.01) and non significantly lower total antioxidant capacity (TAC) and SOD levels than the controls (r = −0.5 and P = 0.02, respectively) denoting the effect of increased oxidative stress. Improvements of the studied oxidative stress parameters were detected after antioxidant administration with significant lowering of MDA levels (P < 0.01) and significant elevation in TAC (P < 0.01) and SOD levels (P < 0.01) [Table 2, Figure 1].
| Variable | Group I beforeAOX (n=17) | Group I afterAOX (n=17) | ||||
|---|---|---|---|---|---|---|
| Appetite | Poor appetite | Improved appetite | ||||
| Physical | Easy fatigability | Increased | ||||
| fitness | and decreased | physical fitness | ||||
| physical fitness | ||||||
| Attacks of | 1-2 attacks/month | One attack/2-3 | ||||
| chest infection | months | |||||
| Petechiae and | Present | Improved | ||||
| ecchymosis | ||||||
| Attacks of | 1-2 attacks/month | One attack/3-4 | ||||
| bleeding | months | |||||
| Rate of blood | 1-2 times/month | 0-3 times/6 | ||||
| transfusion | months | |||||
AOX: Antioxidant
Table 2: Comparison of the clinical manifestations before and after antioxidants intake in the group I (FA)
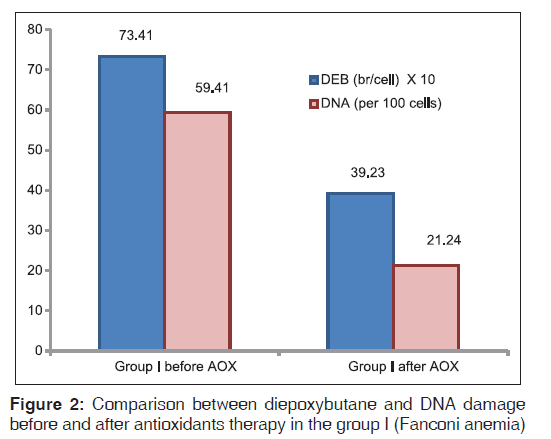
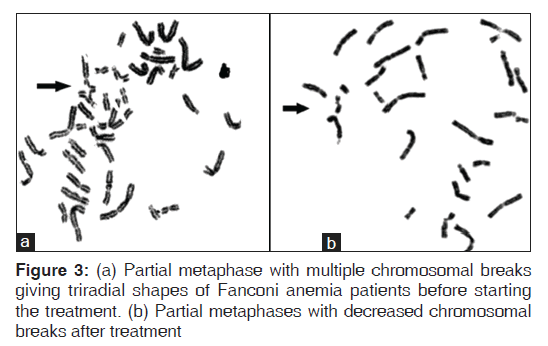
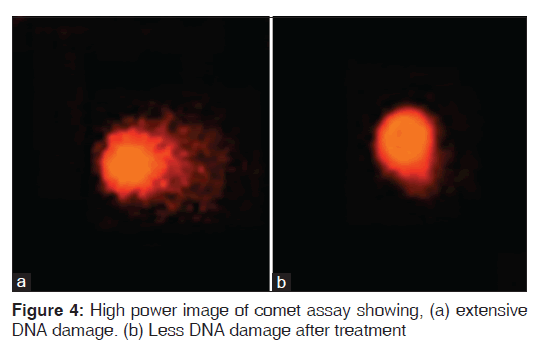
Fanconi anemia group of patients showed more significantly higher DEB-induced chromosomal breakage (P < 0.01) and more DNA damage (P < 0.01) than controls (P < 0.05). Significant improvements of DEB and DNA damage levels were detected after antioxidant administration [Figures 2-4].
Anthropometric measurements including height, weight, and head circumference were done for all FA patients. Analysis of the measurements compared to controls revealed a significant difference in the body weight (P = 0.02), and head circumference (P = 0.04). These measurements were repeated after treatment and revealed non significant improvement mainly in weight. After antioxidants intake, the FA patients showed an improvement of red blood cells, hemoglobin and platelets levels (the most significant improvement was in platelets, P = 0.02), while no change occurred in white blood cells count.
The second group (patients with DS) comprised of 12 males and three females with a ratio of 4:1. Their ages ranged from 1 to 5 years; mean age was 3 (1.36) years. Positive parental consanguinity was observed in 46.6% of cases. There was no family history in DS patients. Congenital heart defect was found in 46.7% patients. Short stature was detected in 33.3% patients, microcephaly in 33.3% patients, and underweight in 20% patients. The body measurements in DS patients compared to the controls revealed a significant difference in the body weight (P < 0.01), height (P < 0.01), and head circumference (P < 0.01).
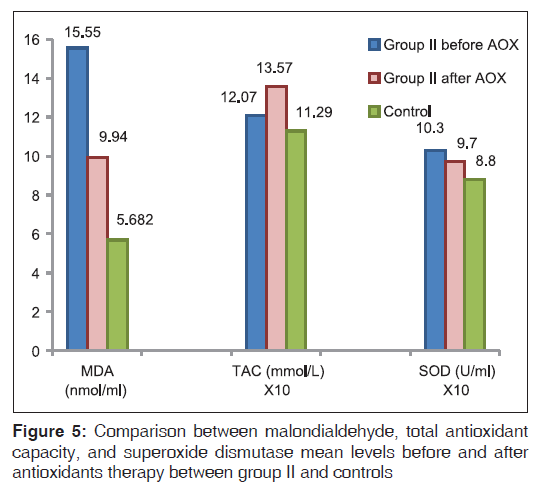
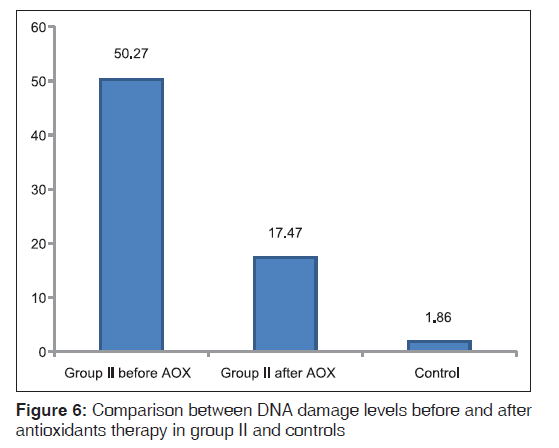
Oxidative stress parameters in DS patients revealed significantly higher MDA (P < 0.01) and SOD (P = 0.01) levels, and non significantly different in TAC levels compared with controls [Figure 5]. A positive correlation was found between SOD and MDA in DS patients (r = 0.53 and P = 0.04, respectively). An improvement in oxidative stress parameters occurred after antioxidant administration with lowering of MDA (P < 0.01) and SOD levels (P < 0.05) state the actual P value and elevation in TAC levels (P < 0.01) [Figure 5]. DS patients showed more significantly higher DNA damage (P < 0.01) than controls. DNA damage levels improved (P < 0.01) non significantly after antioxidant administration [Table 3, Figure 6].
| Variable | Group II beforeAOX (n=15) | Group II afterAOX (n=15) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Generalized hypotonia | Present | Improved | |||||||||
| Developmental milestones | Delayed | Improved | |||||||||
| Attacks of chest infection | 1-2 attacks/month | One attack/ | |||||||||
| 2-3 months | |||||||||||
AOX: Antioxidant
Table 3: Comparison between the clinical manifestations before and after antioxidants intake in group II (down syndrome)
Anthropometric measurements including height, weight, and head circumference were done for all DS patients. Analysis of measurements revealed non-significant improvement mainly in weight.
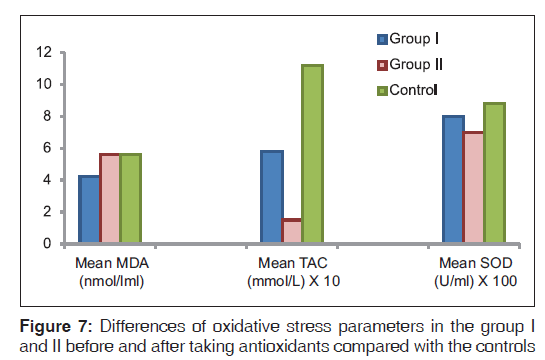
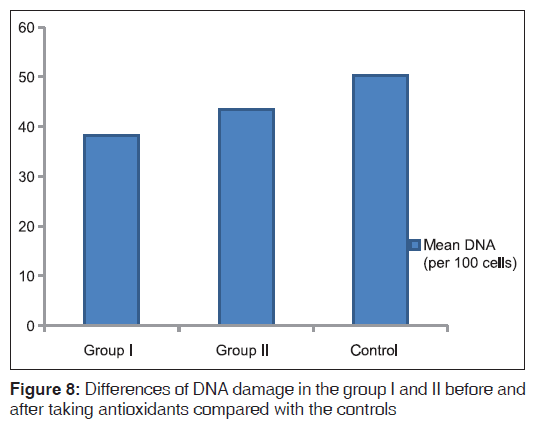
Comparison of the degree of improvement between FA and DS patients after antioxidant intake revealed more improvement in TAC in FA patients (P < 0.01), while a similar degree of improvement occurred in the rest of parameters in both FA and DS patients [Figures 7 and 8].
Discussion
Fanconi anemia and DS are two disorders that show chromosomal aberrations and increase cancer proneness. Understanding the relationship between oxidative stress and the manifestations and progression of these diseases is very crucial.
The first group included in this study was diagnosed as FA by DEB test. Positive parental consanguinity was found in 88.2% of the studied cases. Salem et al.,[23] reported a higher incidence of consanguinity (100%) among previously studied Egyptian FA patients. Other similarly affected family members were present in 58.8% of patients. These findings agree with the autosomal recessive inheritance. Online Mendelian Inheritance in Man [4] reviewed FA and confirmed an autosomal recessive trait in FA.
Anthropometric measurements including height, weight, and head circumference were assessed in the FA group of patients. Analysis of the measurements compared to controls revealed a significant difference in the body weight and head circumference, denoting the effect of FA on the growth of patients. Re-measurements of anthropometry after 6 months of antioxidant supplement showed improvement in most of the patients’ body weight. Slight improvement in height and head circumference was also noticed. This improvement could be due to many factors affecting patient’s growth such as improved appetite, decreased frequencies of chest infections, decreased attacks of bleeding, and good compliance to treatment regimen. Pagano et al.,[24] suggested that antioxidants administration in FA patients aimed at delaying the clinical complications related to oxidative stress. In the present study, oxidative stress parameters in FA patients revealed significantly higher MDA levels than the controls; while SOD was non significantly lower than the controls. On the other hand, there was a non significant difference in TAC levels compared to controls. In addition, a negative correlation was present between SOD and MDA. These findings denote that these patients suffer from the effect of increased oxidative stress. These findings also point to a pro-oxidant state as a key event in the clinical phenotype of FA, providing mechanistic links between oxidative stress and disease progression. Pagano et al.,[24] reported significantly lower SOD activity in FA patients than in the controls. Petrovic et al.,[25] demonstrated the increase of MDA and strongly suggested that FA cells experience increased oxidative stress.
After antioxidants intake, the FA patients showed an improvement of red blood cells, hemoglobin and platelets levels (the most significant improvement was in platelets, while no change occurred in white blood cells count. Improving the oxidative stress status in patients may enhance the hematological cell synthesis and decrease apoptotic process of cells. Ponte et al.,[26] demonstrated that Vitamin E decreases the frequency of chromosomal damage and consequently ameliorating the hematological response.
Fanconi anemia group of patients showed higher DEB-induced chromosomal breakage, and more DNA damage compared to controls. This coincides with the findings of other researches who observed more DNA damage in FA patients than controls.[27,28]
Pagano et al.,[29] determined a direct association between oxidative DNA damage and chromosomal instability as hallmarks of FA phenotype with a significant increase in oxidative stress. In our study, a non significant correlation was found between oxidative stress and each of DEB-induced chromosomal breakage and DNA damage within FA group. This difference may be due to the smaller sample size of our study or may be due to the variation in the individual capacity of the patients to repair the DNA damage.
In the present study, comparing the results of DEB and DNA damage levels in FA patients before and after antioxidants administration, revealed improvement in the level of DEB induced chromosomal breakage, and DNA damage levels. Moreover, improvement in oxidative stress parameters occurred after antioxidant administration with significant lowering of MDA levels and significant elevation in TAC and SOD levels. Various studies regarding the administration of antioxidants to FA patients showed similar improvements of DNA damage and oxidative stress. Astley et al.,[30] concluded in their study that carotenoids and carotenoid-rich foods can influence DNA damage by counteracting oxidative stress. Similarly, Sacheck et al., and Bernard et al.,[28,31] published lower levels of DNA damage after ingestion of 100 mg Vitamin E/day for 12 weeks.
Pincheira et al.,[32] documented that Vitamin E decreased the frequency of chromosomal damage in FA cells. Collins [12] investigated the effect of carotene on oxidative DNA damage and found a negative correlation between total serum carotenoids and oxidized pyrimidines. On the contrary, Giovannelli et al.,[33] did not find a correlation between oxidative damaged DNA and antioxidant intake.
Tiano et al.,[34] reviewed the long-term supplementations with repeated ingestions of antioxidants in tablets or foods. They found improved DNA damage assessed by the comet or 8-OHdG measurement. They suggested that combined supplementation of Vitamin C and Vitamin E has been more successful than the single supplementation in showing a protective effect on oxidative damaged DNA.
The second group included 15 patients with trisomy 21. Congenital heart defect was found in 46.7% patients which was in agreement with the results of Weijerman et al.,[35] who found congenital heart defect in 43% of their DS cases.
Anthropometric measurements including height, weight, and head circumference for all DS patients revealed short stature in 33.3% patients, microcephaly in 33.3% patients and underweight in 20% patients. On the other hand, the body measurements in DS patients compared to the controls revealed a significant difference in the body weight, height, and head circumference. These results are in agreement with Chávez et al.,[36] who elucidated that patients with DS showed a significant decrease in height and weight compared to normal individuals. These results are also matching with an interesting study done by Afifi et al.[37]
Oxidative stress parameters in DS patients revealed significantly higher MDA and SOD levels than the controls, while TAC levels were non significantly comparable to the controls. Furthermore, a positive correlation was found between SOD and MDA in DS patients. These findings confirmed that DS patients are suffering from oxidative stress which is partly due to the over-expression of the SOD gene on chromosome 21 in DS patients.[10] This is in agreement with Garaiová et al.,[38] who detected increased activity of SOD and higher MDA levels than the controls. Campos et al.,[39] proved that TAC was decreased in DS patients. Besides, TAC seems to provide more reliable information about the antioxidant status, at least in DS. In addition, Casado et al.,[40] reported a higher activity of SOD in blood cells of patients with DS compared to controls. They also documented a significant difference in serum concentration of MDA in DS patients.
In the present study, the DS group of patients showed more DNA damage than controls but no correlation was found between DNA damage and oxidative stress parameters. Many studies regarding increased DNA damage in DS are in agreement with the observations of the present study.[8,11,41]
Musalmah et al., (2006) and Ponte et al.,[25,42] reported an increase in DNA damage in lymphocytes of individuals with DS compared with controls, which was reduced by more than 50% after the addition of Vitamin E to the cell culture. As Vitamin E is a powerful antioxidant, they hypothesized that the increased DNA damage in DS resulted from increased oxidative stress with a protective effect of Vitamin E on chromosomal damage.
An improvement in oxidative stress parameters occurred after antioxidant administration with lowering of MDA and SOD levels and elevation in TAC levels. Furthermore, DNA damage levels in DS patients improved after antioxidants intake. Ciaccio et al., Mustafa et al., Muchová et al.,[43-45] Several investigators evaluated the biochemical responses of a nutritional regimen supplemented with vitamins in children with DS and found improvement in the measurements of antioxidant enzymes and reactive oxygen species.
Comparing the degree of improvement of oxidative stress parameters after antioxidants intake between group I and II revealed significant improvement in TAC of group I compared to group II reflecting the more beneficial effect of antioxidants on FA patients. On the other hand, a similar degree of improvement was found in each of MDA and SOD in group I and II. In addition, comparing the degree of improvement of DNA damage after antioxidants intake between group I and II showed no difference. Lloret et al. and Ponte et al.,[2,25] published similar results and suggested that oxidative stress increases genomic instability.
Conclusion
Oxidative stress and DNA damage play a major role in FA and DS pathogenesis. Improvement in oxidative stress parameters and DNA damage occurred after the intake of antioxidants. Early administration of antioxidants should be recommended as a potential therapy for each of FA and DS patients with the ultimate goal of ameliorating life quality and life expectation.
Acknowledgments
The present research is self-funded.
References
- Sulthana SM, Kumar SN, Sridhar MG, Bhat BV, Rao KR. Levels of non enzymatic antioxidants in Down syndrome. Indian J Pediatr 2012;79:1473-6.
- Lloret A, Calzone R, Dunster C, Manini P, d’Ischia M, Degan P, et al. Different patterns of in vivo pro-oxidant states in a set of cancer- or aging-related genetic diseases. Free Radic Biol Med 2008;44:495-503.
- Pagano G, Talamanca AA, Castello G, Pallardó FV, Zatterale A, Degan P. Oxidative stress in Fanconi anaemia: From cells and molecules towards prospects in clinical management. Biol Chem 2012;393:11-21.
- OMIM: Online Mandelian Inheritance in Man. Center for Medical Genetics, John Hopkins University (Baltimore M.D.) and National Center for Biotechnology Information, National Library of Medicine; 2014, May 15. Available from: http:// www.ncbi.nih.gov/OMIM.
- Pagano G, Korkina LG. Prospects for nutritional interventions in the clinical management of Fanconi anemia. Cancer Causes Control 2000;11:881-9.
- Ponte F, Sousa R, Carvalho F, Porto B. Fanconi anemia patients. Toxicol Lett 2011;205:S108.
- Federman N, Sakamoto KM. Topics in pediatric leukemia – Fanconi’s anemia: New insights. Med Gen Med 2005;7:23.
- Morawiec Z, Janik K, Kowalski M, Stetkiewicz T, Szaflik J, Morawiec-Bajda A, et al. DNA damage and repair in children with Down’s syndrome. Mutat Res 2008;637:118-23.
- Lockrow JP, Fortress AM, Granholm AC. Age-related neurodegeneration and memory loss in Down syndrome. Curr Gerontol Geriatr Res 2012;2012:463909.
- Lott IT. Antioxidants in Down syndrome. Biochim Biophys Acta Mol Basis Dis 2012;1822:657-63.
- Savina NV, Smal MP, Kuzhir TD, Egorova TM, Khurs OM, Polityko AD, et al. Biomarkers for genome instability in some genetic disorders: A pilot study. Biomarkers 2012;17:201-8.
- Collins AR. The comet assay for DNA damage and repair: Principles, applications, and limitations. Mol Biotechnol 2004;26:249-61.
- Pallardó FV, Lloret A, Lebel M, d’Ischia M, Cogger VC, Le Couteur DG, et al. Mitochondrial dysfunction in some oxidative stress-related genetic diseases: Ataxia-Telangiectasia, Down Syndrome, Fanconi Anaemia and Werner Syndrome. Biogerontology 2010;11:401-19.
- Zis P, Dickinson M, Shende S, Walker Z, Strydom A. Oxidative stress and memory decline in adults with Down syndrome: Longitudinal study. J Alzheimers Dis 2012;31:277-83.
- Tanner JM, Hiernaux J, Jarman S. Growth and physique studies. In: Weiner JS, Lourie JA, editors. Human Biology: A Guide to Field Methods. IBP Handbook No. 9. Oxford: Blackwell, International Biological Program; 1969. p. 2-71.
- Verma RS, Babu A. Tissue culture techniques and chromosome preparation. In: Verma RS, Babu A, editors. Human Chromosomes Principles and Technique. 2nd ed. New York: McGraw-Hill; 1995. p. 6-71.
- Auerbach AD. Diagnosis of Fanconi anemia by diepoxybutane analysis. In: Dracopoli NC, Haines JL, Korf BR, Moir DT, Morton CC, Seidman CE, et al., editors. Current Protocols in Human Genetics. Vol. 37, Ch. 8. Hoboken, NJ: John Wiley and Sons, Inc.; 2003. p. 1-15.
- Kutler DI, Singh B, Satagopan J, Batish SD, Berwick M, Giampietro PF, et al. A 20-year perspective on the International Fanconi Anemia Registry (IFAR). Blood 2003;101:1249-56.
- Olive PL, Banáth JP. The comet assay: A method to measure DNA damage in individual cells. Nat Protoc 2006;1:23-9.
- Tsukahara H, Jiang MZ, Ohta N, Sato S, Tamura S, Hiraoka M, et al. Oxidative stress in neonates: Evaluation using specific biomarkers. Life Sci 2004;75:933-8.
- Koracevic D, Koracevic G, Djordjevic V, Andrejevic S, Cosic V. Method for the measurement of antioxidant activity in human fluids. J Clin Pathol 2001;54:356-61.
- Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: The good, the bad, and ugly. Am J Physiol 1996;271:C1424-37.
- Salem AM, El-Bassyouni HT, El-Kamah GY, Zarouk WA, Eid MM, Mosaad RM, et al. Screening for common mutations in four FANCA gene exons in Egyptian Fanconi anemia patients. Middle East Journal of Medical Genetics 2014; 3:24–30.
- Pagano G, Talamanca AA, Castello G, d’Ischia M, Pallardó FV, Petrovic S, et al. From clinical description, to in vitro and animal studies, and backward to patients: Oxidative stress and mitochondrial dysfunction in Fanconi anemia. Free Radic Biol Med 2013;58:118-25.
- Petrovic S, Leskovac A, Kotur-Stevuljevic J, Joksic J, Guc-Scekic M, Vujic D, et al. Gender-related differences in the oxidant state of cells in Fanconi anemia heterozygotes. Biol Chem 2011;392:625-32.
- Ponte F, Sousa R, Fernandes AP, Gonçalves C, Barbot J, Carvalho F, et al. Improvement of genetic stability in lymphocytes from Fanconi anemia patients through the combined effect of a-lipoic acid and N-acetylcysteine. Orphanet J Rare Dis 2012;7:28.
- Maluf SW, Erdtmann B. Genomic instability in Down syndrome and Fanconi anemia assessed by micronucleus analysis and single-cell gel electrophoresis. Cancer Genet Cytogenet 2001;124:71-5.
- Bernard ME, Kim H, Berhane H, Epperly MW, Franicola D, Zhang X, et al. GS-nitroxide (JP4-039)-mediated radioprotection of human Fanconi anemia cell lines. Radiat Res 2011;176:603-12.
- Pagano G, Degan P, d’Ischia M, Kelly FJ, Pallardó FV, Zatterale A, et al. Gender and age-related distinctions for the in vivo prooxidant state in Fanconi anaemia patients. Carcinogenesis 2004;25:1899-909.
- Astley SB, Elliott RM, Archer DB, Southon S. Evidence that dietary supplementation with carotenoids and carotenoid-rich foods modulates the DNA damage: Repair balance in human lymphocytes. Br J Nutr 2004;91:63-72.
- Sacheck JM, Milbury PE, Cannon JG, Roubenoff R, Blumberg JB. Effect of Vitamin E and eccentric exercise on selected biomarkers of oxidative stress in young and elderly men. Free Radic Biol Med 2003;34:1575-88.
- Pincheira J, Bravo M, Santos MJ, de la Torre C, López-Sáez JF. Fanconi anemia lymphocytes: Effect of DL-alpha-tocopherol (Vitamin E) on chromatid breaks and on G2 repair efficiency. Mutat Res 2001;461:265-71.
- Giovannelli L, Saieva C, Masala G, Testa G, Salvini S, Pitozzi V, et al. Nutritional and lifestyle determinants of DNA oxidative damage: A study in a Mediterranean population. Carcinogenesis 2002;23:1483-9.
- Tiano L, Padella L, Santoro L, Carnevali P, Principi F, Brugè F, et al. Prolonged coenzyme Q10 treatment in Down syndrome patients: Effect on DNA oxidation. Neurobiol Aging 2012;33:626.e1-8.
- Weijerman ME, van Furth AM, van der Mooren MD, van Weissenbruch MM, Rammeloo L, Broers CJ, et al. Prevalence of congenital heart defects and persistent pulmonary hypertension of the neonate with Down syndrome. Eur J Pediatr 2010;169:1195-9.
- Chávez CJ, Ortega P, Leal J, D’Escrivan A, González R, Miranda LE. Vitamin A deficiency and nutritional status in patients with Down’s syndrome. An Pediatr (Barc) 2010;72:185-90.
- Afifi HH, Aglan MS, Zaki ME, Thomas MM, Tosson AM. Growth charts of Down syndrome in Egypt: A study of 434 children 0-36 months of age. Am J Med Genet A 2012;158A: 2647-55.
- Garaiová I, Muchová J, Šustrová M, Blažíček P. The relationship between antioxidant systems and some markers of oxidative stress in persons with Down syndrome. Biol Bratislava 2004;59:787-94.
- Campos C, Guzmán R, López-Fernández E, Casado A. Urinary uric acid and antioxidant capacity in children and adults with Down syndrome. Clin Biochem 2010;43:228-33.
- Casado A, López-Fernández ME, Ruíz R. Lipid peroxidation in Down syndrome caused by regular trisomy 21, trisomy 21 by Robertsonian translocation and mosaic trisomy 21. Clin Chem Lab Med 2007;45:59-62.
- Tiano L, Littarru GP, Principi F, Orlandi M, Santoro L, Carnevali P, et al. Assessment of DNA damage in Down Syndrome patients by means of a new, optimised single cell gel electrophoresis technique. Biofactors 2005;25: 187-95.
- Musalmah M, NorIsnida I, Shuib S, Gapor MT, Wan Ngah WZ. Gamma-tocotrienol pretreatment reduces DNA damage in lymphocytes of normal subjects and patients with Down syndrome. Malays J Biochem Mol Biol 2006;13:18-21.
- Ciaccio M, Piccione M, Giuffrè M, Macaione V, Vocca L, Bono A, et al. Amino acid profile and oxidative status in children affected by Down syndrome before and after supplementary nutritional treatment. Ital J Biochem 2003;52:72-9.
- Mustafa Nachvak S, Reza Neyestani T, Ali Mahboob S, Sabour S, Ali Keshawarz S, Speakman JR. a-Tocopherol supplementation reduces biomarkers of oxidative stress in children with Down syndrome: A randomized controlled trial. Eur J Clin Nutr 2014;68:1119-23.
- Muchová J, Zitnanová I, Duracková Z. Oxidative stress and Down syndrome. Do antioxidants play a role in therapy? Physiol Res 2014;63:535-42.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.