Pentoxifylline Plus Prednisolone versus Pentoxifylline Only for Severe Alcoholic Hepatitis: A Randomized Controlled Clinical Trial
- *Corresponding Author:
- Dr. Sanjay Kumar Mandal
Geetanjali Apartment, 86/13, Brojen Mukherjee Road, Kolkata - 700 034, West Bengal, India.
E-mail: sanjaypgcal@yahoo.co.in
Abstract
Background: Prednisolone and pentoxifylline (PTX) have been shown to be individually useful in severe alcoholic hepatitis with Maddrey discriminant function (MDF) score ≥32. Previous report suggests that PTX is probably superior to prednisolone alone. However the efficacy of PTX and prednisolone combination over PTX alone in the management of acute alcoholic hepatitis (MDF score ≥32) is yet unrevealed. Aim: The present study was initiated to find out the efficacy of combined pentoxifylline and prednisolone versus PTX alone in acute alcoholic hepatitis in respect of short and intermediate term outcomes. Subjects and Methods: A total of 124 patients with severe alcoholic hepatitis (MDF score ≥ 32) initially were evaluated. 62 patients who fulfilled the inclusion and exclusion criteria were randomized and divided into 2 groups. Group 1 received PTX only, whereas Group 2 received PTX plus Prednisolone. The total duration of follow‑up was 12 months. Student’s t‑test, Chi‑square test, the Kaplan‑Meier methods were used for statistical analysis. Results: A total of 60 patients, 30 in each group were available for final analysis. In Group‑1, 6 patients expired at the end of 1 year (5 within 3 months and another after 3 months). In Group 2, 10 patients expired at the end of 1 year (9 within 3 months and another after 3 months). Though survival probability is higher among Group 1 patients but the difference is not statistically significant. Conclusion: The combination of PTX plus Prednisolone yields no additional benefit in terms of mortality and morbidity from that of PTX monotherapy
Keywords
Alcoholic hepatitis, Maddrey discriminant function score, Pentoxifylline, Prednisolone
Introduction
Alcohol abuse leading to alcoholic liver disease (ALD) is one of the most important causes of preventable morbidity and mortality in the world. Alcohol abuse is responsible for about 50% cases of liver cirrhosis.[1,2] As a consequence, alcoholic cirrhosis is one of the most common indications for liver transplantation in Europe and in North America.[3,4]
ALD is a spectrum that ranges from fatty liver to alcoholic steatohepatitis (ASH) and eventually cirrhosis. Simple hepatic steatosis is the most common histological finding and occurs in 90% of heavy drinkers but is rapidly reversible with abstinence. Alcoholic hepatitis or ASH occurs in up to 35% of heavy drinkers and is usually a precursor of cirrhosis.[5] ASH is a clinico-pathological syndrome that denotes hepatocellular necrosis and inflammation. Pathologic characteristics include development of Mallory “alcoholic” hyaline, prominent intra-sinusoidal collagen deposition and infiltration with polymorphonuclear leukocytes of the hepatic parenchyma, usually at the peri-venular area. However histologically, the majority of patients with severe alcoholic hepatitis have either significant fibrosis or cirrhosis of the liver. Hence alcoholic hepatitis is often superimposed on chronic liver disease and is one of the most important causes of acute on chronic liver failure.[6]
The pathogenesis of liver injury in acute alcoholic hepatitis remains elusive. Genetic factors and immune-mediated mechanisms have been postulated along with a direct hepatotoxic effect of alcohol.[7] Impaired immune response, endoplasmic reticulum stress, mitochondrial dysfunction and free-radical injury induced by alcohol and its acetaldehyde adduct metabolites, Kupffer cell activation and cytokine production, have important roles in accentuating the hepatocyte injury and disease precipitation.[8,9] Studies have shown a linear relationship between tumor necrosis factor-alpha (TNF-α) receptors and mortality from acute alcoholic hepatitis.[10]
Glucocorticosteroids represent the most widely accepted but yet the most debatable therapy in patients with severe ALD. Corticosteroids have been shown to reduce cytokine production, suppress the formation of acetaldehyde adduct metabolites and neo-antigens and inhibit the production of collagen.[11] However down-regulation of steroid sensitivity, risk of infection and a rational therapeutic strategy of corticosteroids are all crucial aspects that need evaluation.
Pentoxifylline (PTX), a non-specific phosphodiesterase inhibitor with anti-inflammatory (TNF-α inhibition) and anti-fibrogenic properties, has been found to be useful in patients with acute alcoholic hepatitis with maddrey discriminant function (MDF) score ≥32.[12,13] Studies have shown that it also has a significant role in decreasing the risk of developing hepatorenal syndrome.[14,15] The beneficial effects are believed to occur through down regulation of TNF-α, interleukin (IL)-1, IL-6, transforming factor-beta, interferon-gamma, stellate cell activation and procollagen I messenger ribonucleic acid (mRNA) expression.[16]
Prednisolone and PTX have been shown to be individually useful in severe alcoholic hepatitis with DF score ≥32.[14,17] A previous trial performed by our team had shown some evidence that PTX is probably superior to prednisolone alone.[15] However the efficacy of PTX and Prednisolone combination over PTX alone in the management of acute alcoholic hepatitis (MDF score ≥32) is yet unrevealed. The present study was initiated to probe into this issue and to measure the short and intermediate term outcomes of such therapeutic interventions.
Subjects and Methods
Totally 124 chronic alcoholic patients attending the liver clinic, outpatient department or emergency medical services of the Medical College and Hospital, Kolkata, were initially considered. The duration of the study was from January 2010 to August 2012. Patients were initially examined clinically, evaluated and subsequently were admitted for the duration of the study. The study protocol was approved by the institutional ethical committee. All the patients underwent investigations for liver chemistry (liver function test, prothrombin time), complete hemogram, random blood sugar, urea, creatinine, electrolytes, viral markers such as hepatitis B surface antigen, anti-hepatitis C virus antibody, hepatitis A virus immunoglobulin M (IgM), hepatitis E virus IgM, serum ceruloplasmin, 24-h urinary copper (as and when required), anti-human immunodeficiency virus (HIV) antibody, chest X-ray, antinuclear antibody, upper gastrointestinal (G.I.) endoscopy and Doppler abdominal ultrasound (as and when required). Patients who had a history of chronic alcohol intake of more than 50 g/day with clinical and biochemical features of severe alcoholic hepatitis (MDF score ≥32 and Aspartate aminotransferase [AST]: Alanine aminotransferase [ALT] >2:1 with absolute value of AST < 500 I.U/L and ALT < 200 IU/L) were included.
ceruloplasmin, 24-h urinary copper (as and when required), anti-human immunodeficiency virus (HIV) antibody, chest X-ray, antinuclear antibody, upper gastrointestinal (G.I.) endoscopy and Doppler abdominal ultrasound (as and when required). Patients who had a history of chronic alcohol intake of more than 50 g/day with clinical and biochemical features of severe alcoholic hepatitis (MDF score ≥32 and Aspartate aminotransferase [AST]: Alanine aminotransferase [ALT] >2:1 with absolute value of AST < 500 I.U/L and ALT < 200 IU/L) were included.
Model for end-stage liver disease (MELD) score and Glasgow alcoholic hepatitis score (GAHS) and Child-Pugh score were calculated for all patients who were included in the study. Only those patients who gave a prior informed written consent for pharmacotherapy were considered for the final study.
The recruited patients were then divided into 2 groups by a computer generated randomization table. Group 1: Patients receiving PTX only. Group 2: Patients receiving PTX plus prednisolone. Now the pharmacotherapy was started within a week of admission.
Patients in Group 1 received PTX (Trental tablets, sanofi Aventis, Mumbai, India) at a dose of 400 mg thrice daily orally and a placebo tablet in the place of prednisolone for the first 4 weeks.
Patients in Group 2 received prednisolone tablet (Wysolone, Wreath, Mumbai, India) at a dose of 40 mg once daily for 4 weeks and PTX tablets at a dose of 400 mg thrice daily for the same duration.
During the study concomitant treatment with salicylates, non-steroidal anti-inflammatory drugs, budesonide, anti-TNF-α agents, Vitamin E, s-adenosyl methionine or ursodeoxycholic acid were not allowed. The investigator, who allocated the patients to the groups, administered the drugs and collected the clinical and laboratory data, as well as statisticians were all blinded regarding the nature of the pharmacotherapy. All the patients were admitted in the wards of the Department of Medicine, Medical College and Hospital, Kolkata for the initial period of 4 weeks. All investigations such as liver function tests, prothrombin time, electrolytes, renal profile and abdominal ultrasound were repeated after the initial 4 weeks of pharmacotherapy.
After the initial 4 weeks, the study was opened and the patients allocated to different groups were revealed. Patients in Group 1 (PTX) who tolerated the drug well, continued to receive the medication at the same dose for the next 8 weeks and then stopped. After 4 weeks of initial therapy, the dose of prednisolone in Group 2 was tapered by 5 mg/week over a period of 7 weeks and then stopped and received PTX like Group 1 patients. Only those patients who were clinically stable at the end of 4 weeks were discharged and later followed-up in the liver clinic. All the patients were counseled for strict alcohol abstinence at the time of discharge from the hospital.
The patients were reviewed at least once a month in the liver clinic. During follow-up, all the patients were examined clinically and asked about drug compliance, intake of alcohol or potential drug adverse effects. Liver function tests, prothrombin time, renal function test, electrolytes and abdominal ultrasounds were performed as and when required. MDF, MELD, GAHS and Child-Pugh scores were calculated for all the patients during follow-up.
Statistical analysis
Student’s t-test was used for analysis of continuous variables. Chi-square test was used for categorical variables. All results of continuous variables were expressed as mean (SD). Survival curves were estimated according to the Kaplan-Meier method and were compared using the log-rank test. Results were considered statistically significant at P < 0.05. SPSS statistics version 17.0 (developer IBM) software was used for statistical analysis.
Results
A total number of 124 patients were initially evaluated. Out of them, 64 patients who fulfilled the inclusion criteria without any other potential etiology of liver injury or severe co-morbid states were considered. One patient refused to give consent for the study and another one refused admission. 62 patients who fulfilled the inclusion and exclusion criteria and who gave informed written consent were randomized and divided into 2 groups. Group 1 (PTX only) had 31 patients while Group 2 (PTX plus Prednisolone) had 31 patients. The total duration of follow-up was 12 months, with the patients being examined and evaluated in the liver clinic on a monthly basis. One patient in Group 1 developed severe vertigo within 7 days after starting PTX and one patient in Group 2 withdrew voluntarily from the study and hence they were excluded. Hence, a total of 60 patients, 30 in each group, were considered for the final analysis.
The baseline parameters (clinical and biochemical) of the total 60 patients, 30 in each group were comparable and shown in Table 1.
| Parameter | Group 1 (PTX) (n=30) | Group 2 (PTX+prednisolone) (n=30) | P |
|---|---|---|---|
| Age (year) | 41.33 (7.81) | 42.73(0.43) | 0.55 |
| Male: Female | 30:0 | 30:0 | - |
| Ascites | 27 | 28 | 0.64 |
| Hepatic encephalopathy | 10 | 11 | 0.79 |
| Varices | 26 | 25 | 0.72 |
| Maddrey DF score | 56.62(37.57) | 63.14(30.98) | 0.46 |
| MELD score | 20.13 (4.47) | 20.9(3.34) | 0.45 |
| GAHS | 7.7 (1.12) | 7.87(0.9) | 0.53 |
| Child’s score | 11.33 (1.47) | 11.90(1.15) | 0.10 |
| Urea (mg/dL) | 31.57(14.30) | 27.60(8.65) | 0.20 |
| Creatinine (mg/dL) | 1.038 (0.19) | 1.013(0.21) | 0.64 |
| Bilirubin (mg/dL) | 4.76(3.72) | 4.67(1.87) | 0.91 |
| Albumin (g/dL) | 2.85(0.57) | 2.9 (0.63) | 0.75 |
| AST (IU/L) | 117.67 (49.55) | 120.40(31.22) | 0.80 |
| ALT (IU/L) | 42.03(18.84) | 48.09(11.93) | 0.09 |
| INR | 2.07(0.81) | 2.11(0.52) | 0.83 |
AST: Aspartate aminotransferase, ALT: Alanine aminotransferase, PTX: Pentoxifylline, SD: Standard deviation, DF: Discriminant function, MELD: Model for end-stage liver disease, GAHS: Glasgow alcoholic hepatitis score, INR: International normalized ratio
Table 1: Comparison of base line parameters of patients receiving PTX (Group 1) versus those receiving PTX plus prednisolone (Group 2) in the treatment of severe alcoholic hepatitis (mean [SD])
In Group – 1, 6 patients expired at the end of 1 year. Out of the 6 patients lost, three patients succumbed in the first 4 weeks; two expired between 4 weeks to 3 months of therapy, while one expired between 3 months to 1 year of therapy. PTX therapy was stopped prematurely (i.e., within 3 months) in 5 patients because of the development of life-threatening complications, all of them died. Out of 5 patients, 3 patients expired following massive (GI) bleeding, 1 patient was lost of progressive hepatic encephalopathy and 1 patient died of sepsis, not responding to conservative management including antibiotics, fluid therapy etc., 1 patient died of GI bleeding after 3 months of therapy.
In Group – 2, 10 patients expired at the end of 1 year out of these 10 patients, 1 succumbed in the first 4 weeks, 8 more were lost between 4 weeks to 3 months of therapy while 1 more was lost between 3 months and 1 year of therapy. PTX plus prednisolone therapy was stopped prematurely (i.e., within 3 months) in 9 patients due to development of life-threatening complications. Out of these 9 patients, 3 patients developed sepsis and all of them died of septic shock despite of adequate antibiotics and hemodynamic support, 1 patient was lost of progressive hepatic encephalopathy, 2 patients had upper GI bleeding and succumbed to hemodynamic failure, 3 patients died of hepatorenal syndrome, not responding to conservative management. This was in sharp contrast to Group – 1, in which none of the patients developed hepatorenal syndrome. 1 patient died of GI bleeding after 3 months of therapy.
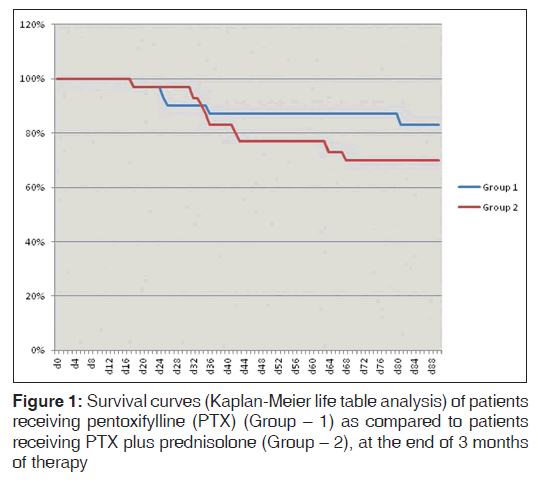
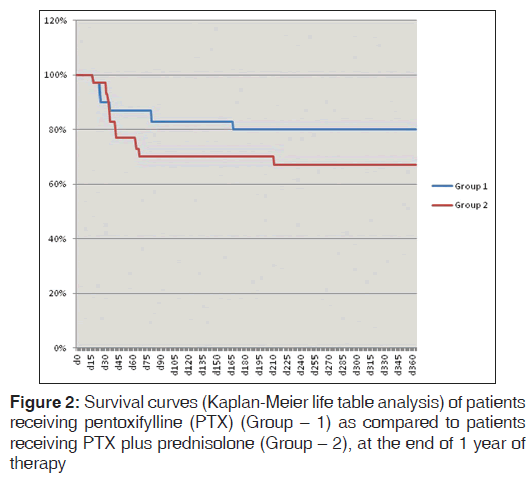
Although, the survival probability was higher among patient receiving only PTX (83.3% [25/30] at the end of 3 months and 80% [24/30] at the end of 1 year) when compared to those receiving PTX plus prednisolone (70% [21/30] at the end of 3 months and 66.7% [20/30] at the end of 1 year), P values were not statistically significant as elaborated in Kaplan-Meier analysis shown in Figures 1 and 2 (P = 0.37 at 3 months and P = 0.32 at 1 year, Log rank test).
Twenty seven patients in Group 1 and 29 patients in Group 2 were evaluated in the liver clinic at the end of 4 weeks. The study was opened at this point in time and the allotment of patients to the different groups was revealed. The investigation done at baseline were repeated and the patients were re-admitted if deemed necessary. The patients were followed-up on a monthly basis and the investigations were repeated at the end of 3 months, 6 months and 1 year. Although the patients did relatively well beyond 3 months of follow-up, only 1 more patient from each group succumbed to the disease.
The morbidity/complication profiles among the two groups were comparable [Table 2]. Nausea followed by vomiting and dyspepsia were the most common adverse effects encountered in both groups. Patients receiving PTX complained of nausea and vomiting more frequently, whereas dyspepsia was more common among those receiving PTX plus prednisolone. Within a week after initiation of PTX, 2 patients from PTX group and 1 patient from PTX plus prednisolone group also developed mild vertigo which subsided spontaneously within few days. Oral thrush, impaired glucose tolerance, poor wound healing were some of the significant problems faced by the patients in the PTX plus prednisolone group.
| Complications | Duration of follow-up | |||
|---|---|---|---|---|
| 0-3 months | 3 months-1 year | |||
| Group 1 (n=30) | Group 2 (n=30) | Group 1 (n=25) | Group 2 (n=21) | |
| Nausea | 20 | 16 | 10 | 3 |
| Vomiting | 10 | 7 | 3 | 1 |
| Dyspepesia | 3 | 8 | 1 | 1 |
| Oral thrush | - | 6 | - | - |
| G.I. bleed | 3 | 2 | 1 | 1 |
| Sepsis | 1 | 3 | - | 2 |
| Recurrent encephalopathy | 1 | 5 | - | - |
| Worsening ascites | - | 1 | - | 1 |
| Impaired glucose tolerance | - | 2 | - | 1 |
| Delayed wound healing | - | 1 | - | 1 |
| Hepatorenal syndrome | - | 3 | - | - |
| Vertigo | 2 | 1 | - | - |
PTX: Pentoxifylline, G.I.: Gastrointestinal
Table 2: Morbidity/complications profile of patients receiving PTX (Group 1) or PTX plus prednisolone (Group 2) in the treatment of severe alcoholic hepatitis
Table 3 shows the baseline profile of patients who succumbed to various complications, as compared to those surviving at the end of the study (i.e., 1 year). It shows that baseline MDF, MELD, GAHS, Child-Pugh score and International Normalized Ratio (INR) were significantly higher among patients who succumbed to the disease as compared to those who survived [P < 0.001, Table 3]. It also shows that baseline albumin was significantly lower among patients who expired as compared to those who survived [P < 0.01, Table 3].
| Parameter | Patients succumbing to complications (n=16) | Surviving patients (n=44) | P |
|---|---|---|---|
| Age (year) | 42.88 (11.82) | 41.73 (8.13) | 0.67 |
| Ascites | 16 | 39 | 0.16 |
| Hepatic encephalopathy | 6 | 15 | 0.81 |
| Maddrey DF score | 94.23(51.30) | 47.39(9.89) | <0.001 |
| MELD score | 24.38 (5.09) | 19.11(2.16) | <0.001 |
| GAHS | 8.69(1.30) | 7.45(0.62) | <0.001 |
| Child’s score | 12.81 (0.91) | 11.18(1.20) | <0.001 |
| Bilirubin (mg/dL) | 6.83(4.24) | 3.95(1.78) | <0.001 |
| Albumin (g/dL) | 2.54(0.64) | 2.99(0.54) | <0.01 |
| AST (IU/L) | 129.63 (60.42) | 115.18(31.39) | 0.23 |
| ALT (IU/L) | 51.94(22.03) | 43.11(12.70) | 0.06 |
| INR | 2.68(1.10) | 1.87(0.16) | <0.001 |
AST: Aspartate aminotransferase, ALT: Alanine aminotransferase, SD: Standard deviation, DF: Discriminant function, MELD: Model for end-stage liver disease, GAHS: Glasgow alcoholic hepatitis score, INR: International normalized ratio
Table 3: Comparison of baseline parameters of patients succumbing to various complications to those surviving at the end of the study (mean [SD])
Table 4 shows the progression of MDF, MELD, Child-Pugh and GAHS score in patients over 12 months. MDF score and MELD score were observed to be significantly lower among patients receiving PTX at the end of 4 week, as compared to those receiving PTX plus prednisolone (P < 0.05 and < 0.01 respectively). But there were no significant differences in MELD, MDF, Child-Pugh and GAHS score between two groups at the end of 3 months, 6 months and 1 year.
| Liver functionscore | Baseline | Duration of follow-up | |||
|---|---|---|---|---|---|
| 28 days | 3 months | 6 months | 1 year | ||
| Maddrey DF Score | |||||
| Group 1 | 56.62(37.57) | 34.89 (8.29) | 23.55 (6.74) | 16.85 (4.69) | 14.80 (5.02) |
| Group 2 | 63.14(30.98) | 43.53 (20.19) | 27.98 (13.11) | 22.40(16.44) | 15.75 (4.31) |
| P value | 0.46 | 0.04 | 0.15 | 0.12 | 0.51 |
| MELD score | |||||
| Group 1 | 20.13 (4.47) | 15.26 (2.22) | 12.56 (1.63) | 10.54 (1.31) | 9.83 (1.52) |
| Group 2 | 20.90 (3.34) | 18.76 (5.70) | 13.76 (3.37) | 11.95 (3.62) | 10.25 (1.51) |
| P value | 0.45 | <0.01 | 0.12 | 0.08 | 0.37 |
| GAHS | |||||
| Group 1 | 7.70(1.11) | 7.04 (0.51) | 6.28 (0.45) | 6.13(0.33) | 6.13 (0.33) |
| Group 2 | 7.87(0.90) | 7.34 (1.11) | 6.57 (0.87) | 6.29(0.78) | 6.15 (0.36) |
| P value | 0.53 | 0.19 | 0.15 | 0.36 | 0.81 |
| Child’s score | |||||
| Group 1 | 11.33 (1.47) | 10.04 (1.69) | 7.88 (1.56) | 6.79(1.41) | 6.58 (1.38) |
| Group 2 | 11.9(1.15) | 10.90 (1.95) | 8.67 (1.74) | 7.10(1.48) | 6.50 (0.82) |
| P value | 0.10 | 0.08 | 0.11 | 0.48 | 0.81 |
PTX: Pentoxifylline, DF: Discriminant function, MELD: Model for end-stage liver disease, GAHS: Glasgow alcoholic hepatitis score
Table 4: Progression of scores evaluating the severity of liver disease of patients receiving PTX (Group 1) as compared to those receiving PTX plus prednisolone (Group 2) in the treatment of severe acute alcoholic hepatitis
Discussion
More than 17 controlled trials and at least 13 meta-analyses have reported the effects of corticosteroids in the treatment of ALD in the past 40 years with conflicting results. However current guidelines of the American College of Gastroenterology recommend the use of glucocorticosteroids in the treatment of patients with severe alcoholic hepatitis as defined by the Maddrey score (DF ≥ 32).[18,19]
PTX, a non-specific phosphodiesterase inhibitor, with combined anti-inflammatory and anti-fibrotic properties, has also emerged as an alternative therapeutic approach. The beneficial effects are believed to occur through various mechanisms such as inhibition of phosphodiesterases, increased cAMP level and down regulation of TNF-α, IL-1, IL-6, TGF-β, interferon-gamma, stellate cell activation and pro-collagen I mRNA expression.[16] It has been shown to block the activation of hepatic stellate cells in culture.[20]
The use of (PTX) in the treatment of severe alcoholic hepatitis has been supported by Akriviadis et al.[14] in a double-blind placebo-controlled trial, which showed that 24% of PTX-treated patients and 46.1% of control patients died during hospitalization. The survival benefit of PTX was found to be related to a significant reduction in the development of hepatorenal syndrome.
Although favorable results of (PTX) used in the treatment of severe alcoholic hepatitis patients with a MDF score ≥32 have been previously reported, it is not currently recommended as a first line treatment for alcoholic hepatitis owing to lack of evidence for its efficacy as compared to the standard treatment with corticosteroids. In a very recent issue of World Journal of Gastroenterology,[15] our team compared for the first time the two treatment modalities head to head in a randomized controlled study, demonstrating the advantage of PTX over corticosteroids in terms of patients’ survival and risk-benefit profile. Prevention of Hepatorenal syndrome may account for the survival advantage of PTX over corticosteroids in patients with severe alcoholic hepatitis.
In the present study, we compared the efficacy of combining PTX to prednisolone over PTX alone. The group receiving PTX and prednisolone had no additional improvement in survival. There was a trend toward reduced survival in the combination group (70% at the end of 3 months and 66.7% at the end of 1 year) when compared to those receiving only PTX (83.3% at the end of 3 months and 80% at the end of 1 year), although statistically non-significant. This increased mortality in PTX plus prednisolone group may be explained by the complications of steroids such as sepsis, GI. bleeding. MDF score in PTX group was significantly lower than PTX plus prednisolone group at the end of 28 days of therapy (34.89 [8.29] in Group 1 vs. 43.53 [20.19] in Group 2, P = 0.04). MELD score in the PTX group was also significantly lower than PTX plus prednisolone group at the end of 28 days of therapy (15.26 [2.22] in Group 1 vs. 18.76 [5.70] in Group 2, P = 0.004). So combination of PTX and prednisolone therapy was no way superior to PTX alone.
One study suggested that patients with a MDF score >54 were at a higher mortality risk from use of steroids than from not being treated.[21] In our study, only 1 patient survived among 9 patients with MDF score >70, those who received PTX plus prednisolone.
Three patients in the PTX plus prednisolone group died due to hepatorenal syndrome but none died of this complication in the PTX group. In spite of increased occurrence of nausea and to a lesser extent vomiting, among patients in the PTX group, they were not severe enough to warrant termination of therapy. Within a week after initiation of PTX, 2 patients from PTX group and 1 patient from PTX plus prednisolone group developed mild vertigo which subsided spontaneously within few days. Oral thrush, impaired glucose tolerance, poor wound healing were some of the significant problems faced by the patients in the PTX plus prednisolone group.
Retrospectively, on analyzing different liver function scores at the time of inclusion, higher MDF, MELD, GASH, Child-Pugh score, INR and low albumin level were associated with the occurrence of increased mortality among patients with severe alcoholic hepatitis.
Limitation of our study is inability to document histological changes in two groups of patients because of unavailability of transjugular liver biopsy in our hospital.
So no therapeutic advantage or mortality benefit was observed by combining PTX with prednisolone. However, further studies with a larger cohort of patients are needed to confirm our findings.
Conclusion
ALD is a major global health burden and newer horizons in the treatment will help better medical control of this disease entity. PTX being one such important molecule has emerged with a promising role. Our present study thus reveals a very important observation that combining these two modalities yields no additional benefit in terms of mortality and morbidity from that of PTX monotherapy.
Source of Support
Nil.
Conflict of Interest
None declared.
References
- Balakrishnan R, Allender S, Scarborough P, Webster P, Rayner M. The burden of alcohol-related ill health in the United Kingdom. J Public Health (Oxf) 2009;31:366-73.
- Méndez-Sánchez N, García-Villegas E, Merino-Zeferino B, Ochoa-Cruz S, Villa AR, Madrigal H, et al. Liver diseases in Mexico and their associated mortality trends from 2000 to 2007: A retrospective study of the nation and the federal states. Ann Hepatol 2010;9:428-38.
- Burra P, Senzolo M, Adam R, Delvart V, Karam V, Germani G, et al. Liver transplantation for alcoholic liver disease inEurope: A study from the ELTR (European Liver Transplant Registry). Am J Transplant 2010;10:138-48.
- Waki K, Tamura S, Sugawara Y, Yamashiki N, Kadowaki T, Kokudo N. An analysis of the OPTN/UNOS liver transplant registry. Clin Transpl 2009;55-64.
- Adachi M, Brenner DA. Clinical syndromes of alcoholic liver disease. Dig Dis 2005;23:255-63.
- Duseja A, Chawla YK, Dhiman RK, Kumar A, Choudhary N, Taneja S. Non-hepatic insults are common acute precipitants in patients with acute on chronic liver failure (ACLF). Dig Dis Sci 2010;55:3188-92.
- Black M, Tavill AS. Corticosteroids in severe alcoholic hepatitis. Ann Intern Med 1989;110:677-80.
- Ji C. Dissection of endoplasmic reticulum stress signaling in alcoholic and non-alcoholic liver injury. J Gastroenterol Hepatol 2008;23 Suppl 1:S16-24.
- Degoul F, Sutton A, Mansouri A, Cepanec C, Degott C, Fromenty B, et al. Homozygosity for alanine in the mitochondrial targeting sequence of superoxide dismutase and risk for severe alcoholic liver disease. Gastroenterology 2001;120:1468-74.
- Spahr L, Giostra E, Frossard JL, Bresson-Hadni S, Rubbia-Brandt L, Hadengue A. Soluble TNF-R1, but not tumor necrosis factor alpha, predicts the 3-month mortality in patients with alcoholic hepatitis. J Hepatol 2004;41:229-34.
- Tome S, Lucey MR. Review article: Current management of alcoholic liver disease. Aliment Pharmacol Ther 2004;19:707-14.
- Agarwal K, Kontorinis N, Kontorinis N, Dieterich DT, Dieterich DT. Alcoholic hepatitis. Curr Treat Options Gastroenterol 2004;7:451-8.
- Haber PS, Warner R, Seth D, Gorrell MD, McCaughan GW. Pathogenesis and management of alcoholic hepatitis. J Gastroenterol Hepatol 2003;18:1332-44.
- Akriviadis E, Botla R, Briggs W, Han S, Reynolds T, Shakil O. Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: A double-blind, placebo-controlled trial. Gastroenterology 2000;119:1637-48.
- De BK, Gangopadhyay S, Dutta D, Baksi SD, Pani A, Ghosh P. Pentoxifylline versus prednisolone for severe alcoholic hepatitis: A randomized controlled trial. World J Gastroenterol 2009;15:1613-9.
- Raetsch C, Jia JD, Boigk G, Bauer M, Hahn EG, Riecken EO, et al. Pentoxifylline downregulates profibrogenic cytokinesand procollagen I expression in rat secondary biliary fibrosis.Gut 2002;50:241-7.
- Maddrey WC, Boitnott JK, Bedine MS, Weber FL Jr, Mezey E, White RI Jr. Corticosteroid therapy of alcoholic hepatitis. Gastroenterology 1978;75:193-9.
- McCullough AJ, O’Connor JF. Alcoholic liver disease: Proposed recommendations for the American College of Gastroenterology. Am J Gastroenterol 1998;93:2022-36.
- Rongey C, Kaplowitz N. Current concepts and controversies in the treatment of alcoholic hepatitis. World J Gastroenterol 2006;12:6909-21.
- Lee KS, Cottam HB, Houglum K, Wasson DB, Carson D, Chojkier M. Pentoxifylline blocks hepatic stellate cell activation independently of phosphodiesterase inhibitory activity. Am J Physiol 1997;273:G1094-100.
- Mendenhall C, Roselle GA, Gartside P, Moritz T. Relationship of protein calorie malnutrition to alcoholic liver disease: A reexamination of data from two Veterans Administration Cooperative Studies. Alcohol Clin Exp Res 1995;19:635-41.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.