Post-Finasteride Syndrome: Current Views and Where do we Stand?
2 Dermatology Department, King Saud Hospital in Unaizah, Qassim, Saudi Arabia
3 Research Center, College of Medicine, Qassim University, Qassim, Saudi Arabia
Received: 24-Dec-2021, Manuscript No. AMHSR-22-48235; Editor assigned: 27-Dec-2021, Pre QC No. AMHSR-22-48235(PQ); Accepted Date: Jan 24, 2022 ; Reviewed: 11-Jan-2022 QC No. AMHSR-22-48235; Revised: 17-Jan-2022, Manuscript No. AMHSR-22-48235(R); Published: 24-Jan-2022, DOI: 10.54608.annalsmedical.2022.23
Citation: Alhetheli GI, et al. Post-Finasteride Syndrome: Current Views and Where do we Stand?. Ann Med Health Sci Res. 2022;12:12-18.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Introduction: Androgenetic alopecia is the most frequent cause of hair loss worldwide, affecting around 70% of males and 40% of females. Since the approval of finasteride for androgenetic alopecia, several studies have reported various psychological and sexual side effects. In 2012, the food and drug organization made changes to the drug insert stating the possibility of persistent side effects, or what is known as post-finasteride syndrome. There is still not much known about the rate of these side effects and the causal relationship. Methods: A literature search was performed using the Pubmed and Google Scholar databases that included studies conducted from 1995 to 2020. Results: There were 47 identified articles in Pubmed, while 152 articles were identified using Google Scholar. Duplicates were removed, leaving a total of 185 articles. Following a second, thorough screening of the titles and abstracts, only 62 full-text articles were reviewed. Of those, 35 articles were chosen to be included. Conclusion: Based on the existing literature, the medical community believes that these patterns of symptoms constitute the basis for PFS in individuals predisposed to epigenetic susceptibility. The medical community must define and characterize the pathophysiological mechanisms underlying PFS, and more attention should be devoted to patient education and counseling as well as to developing novel management modalities. Further highquality clinical studies are needed to evaluate the potential neuropsychiatric side effects of finasteride in humans and to establish whether finasteride has any exact causal relationship with suicidal ideation and other reported side effects.
https://transplanthair.istanbul
https://hairclinicturkey.co
https://hairclinicistanbul.co
https://besthairtransplant.co
https://hairtransplantistanbul.co
Keywords
PFS: Post Finasteride Syndrome; Finasteride adverse effects; Androgenetic alopecia; Male pattern hair loss
Abbreviations
AGA: Andro Genetic Alopecia; MPHL: Male Pattern Hair Loss; FPHL: Female Pattern Hair Loss; 5α-DHT: 5α-Dihydrotestosterone; T: Testosterone; PROG: Progesterone; 5α-R: 5α-Reductase; DHT: Dihydrotestosterone; DHP: Dihydroprogesterone; 3α-HSOR: 3α-Hydroxysteroid Oxidoreductase; 3β-HSOR: 3β-Hydroxysteroid Oxidoreductase; 3α-diol: 3α-androstanediol; 3β-diol: 3β-androstanediol; THP: Tetrahydroprogesterone; PREG: Pregnenolone; DHEA: Dehydroepiandrosterone; PR: Progesterone Receptor; BPH: Benign Prostatic Hyperplasia; PFS: Post-Finasteride Syndrome; FDA: Food and Drug Administration; NIH: National Institute of Health; MDD: Major Depressive Disorder
Introduction
Andro Genetic Alopecia (AGA) is the most frequent cause of hair loss worldwide, affecting around 70% of males as Male Pattern Hair Loss (MPHL) and 40% of females as Female Pattern Hair Loss (FPHL) at some point in their lives. [1,2] It is characterized by progressive non-cicatricial baldness of the scalp vertex and recession of temporal hairlines due to 5α-Dihydrotestosterone (5α-DHT) dependent miniaturization of hair follicles. [3] The enzyme 5α-Reductase (5α-R) plays a crucial role in the activation of neuro active steroids like Testosterone (T) and Progesterone (PROG). Subsequently T and PROG are metabolized by (5α-R) into Dihydrotestosterone (DHT) and Dihydroprogesterone (DHP), respectively. All neuroactive steroids (i.e. steroids synthesized by the peripheral nervous system and also those synthesized directly by the central nervous system) are then further transformed by either 3α-Hydroxysteroid Oxidoreductase (3α-HSOR) or 3β-Hydroxysteroid Oxidoreductase (3β-HSOR) into more metabolites, such as 5α-androstane-3α, 17β-diol (3α-diol) or 5α-androstane-3β, 17β-diol (3β-diol) in case of DHT, and Tetrahydroprogesterone (THP, also known as allopregnanolone) or isopregnanolone in case of DHP. All these neuroactive steroids together with their precursors (i.e. Pregnenolone: PREG and Dehydroepiandrosterone: DHEA), which interact with classical steroid receptors (e.g. Progesterone: PR, Androgen: AR and Estrogen Receptors: ER) and non-classical (e.g. neurotransmitter and membrane steroid receptors) steroid receptors, act as vital modulators of nervous system function. [4,5]
Finasteride, an inhibitor (5α-R), was approved for the treatment of AGA in 1997. [6] Over the years, finasteride has been well tolerated and has a relatively good safety profile with rare and reversible side effects, such as decreased sexual libido and ejaculatory volume, specifically when prescribed in a dose of 5 mg for cases of Benign Prostatic Hyperplasia (BPH). [7] However, there are several reports describing a constellation of persistent sexual, neuropsychiatric and physical side effects that appear or continue after drug discontinuation, inducing what is called Post-Finasteride Syndrome (PFS). Consequently, related authorities in several countries raised warnings related to this drug. In 2012, the Food and Drug Administration (FDA) made changes to the package insert in the United States, stating the possibilities of persistent side effects. [8] In 2015, the National Institute of Health (NIH) categorized PFS under rare and genetic diseases. [9,10]
There is still not much known about the rate of these side effects and the causal relationship, and the results of current studies are insufficient and non-conclusive. This review article aimed to shed light on the genesis of PFS, how common it is and the magnitude of its impact on patients taking it for AGA. It also offers general recommendations on how to avoid and manage the condition.
Methodology
Search strategy
Our literature search was performed using the Pubmed and Google Scholar databases to find articles published from 1995 to 2020. The search keywords used were PFS, post finasteride syndrome, finasteride adverse effects, androgenetic alopecia and male pattern hair loss. We limited our search to articles written in english. The relevance of the information was determined by two independent authors after screening the titles and abstracts. The obtained full-text articles ‘that were found to be relevant’ were reviewed again by the same two authors independently, and a final list of included studies was made.
Study selection
All studies explained the adverse effects of finasteride in androgenetic alopecia, especially in connection with post-finasteride syndrome. Studies explaining the psychological effects of androgenetic alopecia on males were also included.
Data extraction
Two authors screened each study independently and extracted the following data using a standard format: First author’s last name, publication year, and psychiatric and/or sexual features in post-finasteride patients or psychological effects of androgenetic alopecia. A consensus was reached after discussion between the two authors.
Results
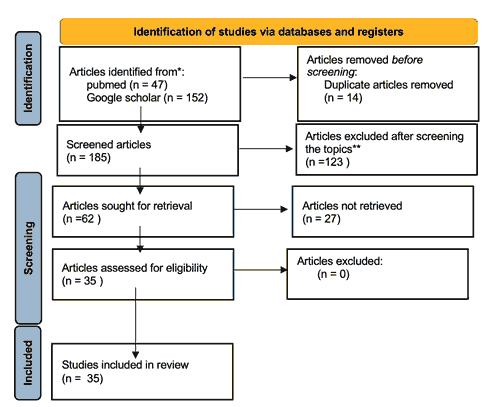
47 articles were identified in Pubmed, while 152 were identified using Google Scholar. Duplicates were removed, leaving a total of 185 articles. Following a second, thorough screening of the titles and abstracts, only 62 full-text articles were reviewed. Of those, 35 articles were chosen to be included in our review. Figure 1 summarizes our identification and selection of relevant studies.
The association between androgenetic alopecia and psychological disorders has been well documented in several studies. Table 1 shows the studies that revealed a negative psychological impact of AGA on affected individuals. These studies agree that there is a greater psychological impact among young males with AGA compared with older adults.
| Source | Year | Psychological effects of androgenetic alopecia in men |
|---|---|---|
| Wells et al. [11] | 1995 | Severe and progressive hair loss has been linked to depression, low confidence and low self-esteem in young men. |
| Cash et al. [12,13] | 1999, 2001 | Hair loss negatively affects the individual’s feelings of attractiveness and satisfaction with his physical appearance and also influences others people’s perceptions of the balding individual. There is considerable preoccupation and moderate distress with significant coping effects. |
| Girman et al. [14] | 1998 | The prevalence of personality disorders was significantly higher in subjects with male pattern hair loss. These personality profiles include the following: more worrying, concern about getting older, perceived noticeability by others and greater dissatisfaction with their hair appearance. These intern were more pronounced in younger men with AGA. |
| Grimalt [15] | 2005 | It is unknown whether AGA is a causal factor in the development of low self-esteem, depression, introversion and feelings of unattractiveness, or whether there are predisposing factors in certain patients prior to developing hair loss. |
| Alfonso [16] | 2005 | Seventy percent of study participants stated that hair has a significant impact on body image, while 62% reported that hair loss could affect self-esteem. Forty-three percent of men reported a decrease in personal attractiveness due to hair loss and 42% stated a fear of becoming bald, while 37% were concerned about getting older. Twenty-two percent and 21% reported negative effects on social life and feelings of depression, respectively. Reduced self-confidence in personal attractiveness was also reported by 38% of men who were not in stable romantic relationships. |
| Molina et al. [17] | 2016 | In men from 18-40 years of age, psychosocial impairment related to dissatisfaction with body image due to hair loss has been linked to an increased risk of sexual dysfunction. |
| Montomery et al. [18] | 2017 | More than a third of the study group with hair loss had clinically significant anxiety, while 15% had severe anxiety and 50% of the men expressed high levels of social anxiety. |
| Ng et al. [19] | 2017 | Males younger than 40 years who were single reported more anxiety and depression than older married patients. Additionally, patients who adopted coping strategies reported more anxiety and depression compared to those who were not using any coping strategies. |
| Gokalp [20] | 2017 | Androgenetic alopecia is associated with anxiety, depression, social phobia, post-traumatic stress disorder and suicidal thoughts. |
| Danyal et al. [21] | 2018 | Men with MPHL suffer from significantly lower self-esteem and higher anxiety compared to men without MPHL. Young men with mild–moderate hair loss have more anxiety than those with moderate–severe hair loss. |
Table 1: Reported psychological effects of androgenetic alopecia in males.
Multiple clinical studies have shown that finasteride administration induces various adverse physical, mental/neurological and sexual effects in some patients that may be either reversible or persistent despite treatment cessation. When these constellations of symptoms last for more than three months after the cessation of medication, it is called post-finasteride syndrome. Table 2 represents the reported psychiatric features of PFS, while Table 3 shows the reported sexual adverse effects seen in former finasteride users for AGA treatment. [11-22]
| Source | Year | Psychiatric features in post-finasteride patients |
|---|---|---|
| Altomare et al. [22] | 2002 | Among 23 subjects who were subjected to finasteride 1mg/day for AGA, 19 patients developed mood disturbances during treatment. |
| Irwig [23] | 2012 | Seventy-five percent of former finasteride users experienced depressive symptoms, which was significantly higher compared to the controls. Moderate or severe depressive symptoms were present in 64% and suicidal thoughts were reported by 44% of participants. |
| Ganzer et al. [24] | 2015 | Nearly three-quarters of respondents reported increased anxiety (74%), depressive effects (73%), emotional flatness (55%), anhedonia (73%), and insomnia (58%), and 63% had experienced negative thoughts and suicidal ideation on a nearly daily basis. |
| Caruso et al. [25] | 2015 | All subjects had complex and constant neuropsychiatric patterns. It was reported that altered levels of neuroactive steroids associated with depressive symptoms are present in AGA patients even after discontinuation of the finasteride treatment. |
| Basaria et al. [26] | 2016 | Symptomatic finasteride users had higher depression scores (reported depressed mood ranged from moderate to severe depression), a more negative affectivity balance and more cognitive complaints than men in groups 2 and 3, but had normal, objectively assessed cognitive function. |
| Melcangi et al. [27] | 2017 | Half of the male patients in the study group suffered from a clinically significant behavioral or psychological syndrome, i.e., major depressive disorder (MDD) and altered levels of neuroactive steroids. PFS patients reported decreased self-confidence, irritability, nervousness, agitation, inner restlessness, depression, hopelessness, feeling of worthlessness, suicidal thoughts, anxiety, panic attacks and sleep problems. Additionally, PFS patients reported cognitive complaints, such as difficulty with concentration, mental confusion, forgetfulness, loss of short-term memory, loss of thought or reasoning and slurred speech. |
| Traish [28] | 2018 | Finasteride alters gene expression, including upregulation of androgen receptors (AR), increased histone acetylation, and methylation of a host of receptors and enzymes that may impair dopaminergic signaling and other neurotransmitter receptors, thus causing persistent or permanent adverse effects manifested in anxiety, depression, and suicidal ideation. |
| Albasher et al. [29] | 2020 | Prominent psychiatric symptoms were depression, anxiety, panic attacks, feelings of isolation and brain fog. Insomnia and fatigue were reported to be the most debilitating symptoms. It was concluded that men under 40 years of age who use finasteride for AGA are at risk for suicide if they develop persistent sexual adverse effects and insomnia. |
Table 2: Psychiatric features reported by former finasteride users linked to the treatment of androgenetic alopecia.
| Source | Year | Sexual features in post-finasteride patients |
|---|---|---|
| Pereira [30] | 2020 | The current data from the literature are controversial, and it is still not possible to establish a causal relationship between 5α-reductase inhibitors and the persistence of sexual symptoms. The studies that showed a higher incidence of persistent sexual side effects due to the use of finasteride have important biases, included limited samples and are insufficient to confirm the existence of PFS. |
| Belknap et al. [3] | 2015 | This meta-analysis reviewed 34 clinical trials reporting the adverse effects of finasteride use in AGA. Odds ratios of sexual dysfunction were calculated for seven studies, decreased libido for 12 studies, and impotence for seven studies. All three funnel plot outcomes of odds ratios. vs sample size showed a bias toward lower odds ratios, suggesting systematic underdetection. |
| Hirshburg et al. [31] | 2016 | The most common complications caused by finasteride use are sexual side effects. Erectile dysfunction was the most common in this category, followed by ejaculatory dysfunction and loss of libido. The persistence of these adverse effects is somewhat controversial. |
| Traish et al. [32] | 2011 | Protracted sexual dysfunction adverse effects like erectile dysfunction and diminished libido are reported by a subset of men, raising the possibility of a causal relationship. |
| Maksym et al. [33] | 2019 | Current literature has reported multiple adverse effects of 5ARIs. However, their true association, causative relation, prevalence, and clinical significance are population-dependent and mostly remain obscured. |
| Giatti et al. [34] | 2018 | This review indicated that persistent sexual dysfunction is a shared feature of Post-Finasteride Syndrome and Post-SSRI Sexual Dysfunction. Neuroactive steroids, serotonin and dopamine are variably interconnected with PSSD and PFS. However, further experiments should explore the synthesis and signaling of these neurotransmitters. |
| Traish [35] | 2020 | The sexual adverse effects of finasteride use are reported in almost all clinical studies at varying degrees of severity. One of the most prominent observations is the increased incidence and persistence of sexual dysfunction (decreased or loss of libido, ED and orgasmic dysfunction), irrespective of duration of study, drug or dose used. |
| Chiriacò et al. [36] | 2016 | Using the ASEX questionnaire, out of 79 participants, 40.5% declared that getting and keeping an erection was very difficult, while 3.8% never achieved one. Reaching orgasm was declared very difficult by 16.5% and never achieved by 2.5%. By the ad hoc questionnaire, the most frequent sexual symptoms were loss of penis sensitivity (87.3%), decreased ejaculatory force (82.2%) and low penile temperature (78.5%). |
| Ganzer et al. [24] | 2014 | Out of 131 surveys filled out by participants, 93% reported decreased sex drive, 63% reported a complete loss of sex drive, 83% reported intermittent erectile dysfunction and 40% reported complete impotence. Eighty-nine percent reported loss of morning and spontaneous erection, while 40% reported failure to achieve orgasm on most occasions and 70% reported sexual anhedonia. Additionally, 82% reported diminished semen volume and force, while 79% reported penile shrinkage and sensory changes. Lastly, 51% reported scrotal shrinkage and numbness. |
| Rezende et al. [37] | 2018 | Persistent sexual side effects were only reported in low-quality studies with strong selection bias, while a significant nocebo effect has been documented among patients informed about the possible adverse effects of finasteride. |
| Basaria et al. [26] | 2016 | The participants were divided into three groups: symptomatic finasteride users (n=25), non-symptomatic finasteride users (n=13) and a healthy control group of non-users (n=18). Men in the first group had significantly lower International Index of Erectile Function (IIEF) composite scores: a median of 30, 67 and 68.8 for groups 1, 2 and 3, respectively. There were significantly lower IIEF scores in each of the erectile function, sexual desire, orgasmic function, intercourse satisfaction, and decreased overall satisfaction categories among men in group 1 than men in groups 2 and 3. |
Table 3: Sexual features reported in post-finasteride syndrome.
Discussion
Finasteride is an orally active specific inhibitor of 5-alpha-reductase, which is an enzyme that blocks the conversion of testosterone to Dihydrotestosterone (DHT). It is widely used to treat Benign Prostatic Hypertrophy (BPH) and Male Pattern Hair Loss (MPHL), which are associated with elevated levels of DHT and 5-a-reductase activity in the prostate and hair follicles, respectively. A study conducted by Irwig et al. showed that a significant proportion of men reported intolerable Adverse Effects (AEs) after initiating finasteride therapy and continued to experience these effects after stopping the medication. [23] The most frequent AEs are classified into physical, sexual, cognitive and psychological effects. Physical AEs include chronic fatigue, gynecomastia, muscle atrophy, skin thinning, and penile and scrotal shrinkage. Decreased libido, intermittent erectile dysfunction and impotence are reported as sexual AEs. Cognitive AEs include brain fog and difficulty with concentrating and maintaining focus. Emotional sensitivity, depression and excessive anxiety are reported as psychological AEs. [24-38]
Walf et al. categorized the AEs following the use and discontinuation of finasteride and found that 32% reported anti androgenic AEs, 19% reported estrogenic AEs, 30% reported central/brain AEs and 5% reported nonspecific/severe AEs. Meanwhile, 14% reported AEs in all categories. These undesirable and persistent AEs are collectively called Post-Finasteride Syndrome (PFS). [39,40] PFS is a condition associated with altered levels of neuroactive steroids and varied AEs that persist after cessation of finasteride treatment. Neurosteroids and neuroactive steroids are produced in the central nervous system from adrenal and gonadal steroids, and the synthesis of these steroids requires 5-alpha-reducatase. These steroids possess antidepressant, anticonvulsant and anxiolytic properties. [41] Depression is shown to be associated with dysregulation of neurosteroids, inhibition of 5-alpha-reductase biosynthesis and androgen deficiency. [32] Reduced 5-a-reductase activity is observed in humans with depressive illness, and finasteride treatment has been linked to an increase of depressive symptoms. More than 80% of patients developed moderate to severe depression during finasteride treatment of 1 mg/day orally for androgenetic alopecia. Impaired socio familial relations and altered sleep and eating behaviors with associated anxiety were reported after 9-29 weeks of finasteride treatment, whereas these symptoms were promptly resolved after drug suspension. Interestingly, a study performed by Altomare reported a relapse of depression within two weeks of re-administration of finasteride treatment in 8% of patients. [22] Other study revealed that seventy five percent of former finasteride users for MPHL showed significantly higher rates of depression and suicidal thoughts than controls (10%). Of those 75%, moderate to severe depressive symptoms were seen in 64% of the finasteride group, while 44% of prior finasteride users experienced suicidal thoughts, which was significantly higher when compared with 3% of controls. [23]
It was also postulated that finasteride treatment affects the brain’s neuronal plasticity on a structural level due to the significant decrease in 5-alpha dihydrotestosterone levels, which induce a reversible reduction in newborn cell numbers and young neurons in the hippocampus seen after finasteride administration. Neurogenesis was found to be reversed to normal 35 days after the last finasteride injection in male mice. These hippocampal neurogenesis changes after finasteride treatment have been linked to emotional behavior and this might contribute to the pathophysiology of affective disorders in humans.
The US Food and Drug Administration (FDA) adverse event reporting system database recorded 36 death cases due to psychiatric side effects of finasteride administration. In 2011, finasteride-related depression and suicidality led to an FDA post-marketing report. [8] Depression was added to the adverse reaction section of finasteride product labels in the USA. Insomnia, fatigue and persistent sexual dysfunction were found to be a pattern of common symptoms among those who committed suicide after finasteride cessation. There was no baseline medical or psychiatric diagnosis prior to starting finasteride. Although associations between medication use and symptoms are not proven, studies have postulated that men under the age of 40 who are taking finasteride for alopecia are at risk for suicide if they develop a pattern of persistent sexual adverse effects and insomnia. According to the pharmaco vigilance case-noncase study of 153 countries in the Vigibase database, global data from the World Health Organization’s individual case safety reports stated that there was a significant Reporting Odds Ratio (ROR) for suicidality; depression and anxiety were associated with finasteride use in male patients younger than 45 years who used it for alopecia. Patients 45 years old or below with alopecia were found to have more than a four times higher risk of depression, suicidal ideation and anxiety while using finasteride when compared with users over 45 years of age and users with BPH.
One large meta-analysis performed by Belknap determined that out of all included clinical trials, none met the criteria for adequate safety reporting. Additionally, no validated, effective and standard methods were used for revealing adverse effects, assessing their severity and determining the causality. This meta-analysis also revealed that among those 34 clinical trials, only one study 48 used a validated method of depression assessment, and it showed a significant association between finasteride use and depression. It concluded that the exact rate of finasteride AEs has yet to be determined by high-quality studies. [3]
Many clinicians are unaware of the scope of the persistent physical and psychological adverse effects of finasteride while it is in usage and despite its discontinuation. The treating clinician should have knowledge and awareness about the higher prevalence of depression and sexual dysfunction in the subset of men seeking treatment for AGA in comparison to the general population. Hence, it is mandatory to ask about patients’ histories of preexisting depression or sexual dysfunction before starting finasteride treatment. Studies agree that 5-alpha reductase inhibitors are well-tolerated, but not without risk. Accordingly, patient education prior to treatment is of the utmost importance, and alternative therapies for treating alopecia may be considered during patient counseling.
Conclusion
Post-Finasteride Syndrome (PFS) is considered a serious group of adverse effects that range between persistent, irreversible sexual, neurological, physical and psychological symptoms that develop and persist in patients during and/or after discontinuing finasteride treatment in men. Based on the existing literature and clinical research, the medical community believes that these patterns of symptoms constitute the basis for PFS in individuals predisposed to epigenetic susceptibility. The medical community must define and characterize the pathophysiological mechanisms underlying PFS, and more attention should be devoted to patient education and counseling as well as to developing novel management modalities. In addition, further high-quality clinical studies are needed to evaluate potential neuropsychiatric side effects of finasteride in humans and to establish whether finasteride has any exact causal relationship with suicide ideation and other reported side effects.
References
- Mc Elwee KJ, Shapiro JS. Promising therapies for treating and/or preventing androgenic alopecia. Skin Ther Lett. 2012;17:1-4. [Crossref], [Google Scholar], [Indexed]
- Belknap SM, Aslam I, Kiguradze T. Adverse event reporting in clinical trials of finasteride for androgenic alopecia: A meta-analysis. JAMA Dermatol. 2015;151:600-606. [Crossref], [Google Scholar], [Indexed]
- Silvia G, Luis M, Barreto GE, Melcangi RC. Neuroactive steroids, neurosteroidogenesis and sex. Prog Neurobiol, 2019;176:1-17. [Crossref], [Google Scholar], [Indexed]
- Melcangi RC, Casarini L, Marino M. Altered methylation pattern of the SRD5A2 gene in the cerebrospinal fluid of post-finasteride patients: A pilot study. Endocr Connect. 2019;8:1118-1125. [Crossref], [Google Scholar], [Indexed]
- Kaufman KD, Olsen EA, Whiting D, Savin R, DeVillez R, Bergfeld W, et al. Finasteride in the treatment of men with androgenetic alopecia finasteride male pattern hair loss study group. J Am Acad Dermatol. 1998;39:578-89. [Crossref], [Google Scholar], [Indexed]
- Cather JC, Lane D, Heaphy MR, Nelson BR. Finasteride an update and review. 1999;64:167-72. [Crossref], [Google Scholar], [Indexed]
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. 2021;372. [Crossref], [Google Scholar]
- Wells PA, Willmoth T, Russell RJ. Does fortune favour the bald? Psychological correlates of hair loss in males. Br J Psychol. 1995;86:337-44. [Crossref], [Google Scholar], [Indexed]
- Cash TF. The psychosocial consequences of androgenetic alopecia: A review of the research literature. Br J Dermatol.1999;141:398-405. [Crossref], [Google Scholar], [Indexed]
- Cash TF. The psychology of hair loss and its implications for patient care. Clin Dermatol. 2001;19:161-166. [Crossref], [Google Scholar], [Indexed]
- Girman CJ, Rhodes T, Lilly FRW, Guo SS, Siervogel RM, Patrick DL, et al. Effects of self-perceived hair loss in a community sample of men. 1998;197:223-229. [Crossref], [Google Scholar], [Indexed]
- Grimalt R. Psychological aspects of hair disease. J Cosmet Dermatol. 2005;4:142. [Crossref], [Google Scholar], [Indexed]
- Alfonso M, Richter AH, Tosti A, Viera MS, García M. The psychosocial impact of hair loss among men: A multinational European study. Curr Med Res Opin. 2005;21:1829-36. [Crossref], [Google Scholar], [Indexed]
- Molina LA, Caparros MI, Gomez AP, Alcalde AM, Jimenez MJJ. Psychosocial impairment as a possible cause of sexual dysfunction among young men with mild androgenetic alopecia: A cross-sectional crowdsourcing web-based study. ADC. 2016;24:42-48. [Crossref], [Google Scholar], [Indexed]
- Montgomery K, White C, Thompson A. A mixed methods survey of social anxiety, anxiety, depression and wig use in alopecia. BMJ Open. 2017;4:e015468. [Crossref], [Google Scholar]
- Ng KF, Norazirah MN, Mazline MB, Adawiyah J, Shamsul AS. Psychological impact, self-perception and the contributing factors in patients with androgenetic alopecia. J Cosmo Trichol. 2017;123:1-7. [Crossref], [Google Scholar]
- Altomare G, Capella GL. Depression circumstantially related to the administration of finasteride for androgenetic alopecia. J Dermatol. 2002;29:665-9. [Crossref], [Google Scholar], [Indexed]
- Irwig MS. Depressive symptoms and suicidal thoughts among former users of finasteride with persistent sexual side effects. J Clin Psychiatry. 2012;73:1220-3. [Crossref], [Google Scholar], [Indexed]
- Ganzer CA, Jacobs AR, Iqbal F. Persistent sexual, emotional, and cognitive impairment post-finasteride: A survey of men reporting symptoms. Am J Mens Health. 2015;9:222-228. [Crossref], [Google Scholar], [Indexed]
- Caruso D, Abbiati F, Giatti S, Romano S, Fusco L, Cavaletti G, et al. Patients treated for male pattern hair with finasteride show, after discontinuation of the drug, altered levels of neuroactive steroids in cerebrospinal fluid and plasma. J Steroid Biochem Mol Biol. 2015;146:74-9 [Crossref], [Google Scholar], [Indexed]
- Basaria S, Jasuja R, Huang G, Wharton W, Pan H, Pencina K, et al. Characteristics of men who report persistent sexual symptoms after finasteride use for hair loss. J Clin Endocrinol Metab. 2016;101:4669-4680. [Crossref], [Google Scholar], [Indexed]
- Melcang RC, Santi D, Spezzano R, Grimoldi M, Tabacchi T, Fusco ML, et al. Neuroactive steroid levels and psychiatric and andrological features in post-finasteride patients. J Steroid Biochem Mol Biol. 2017;171:229-235. [Crossref], [Google Scholar]
- Traish AM. The post-finasteride syndrome: Clinical manifestation of drug-induced epigenetics due to endocrine disruption. Curr Sex Health Rep. 2018;10:88-103. [Crossref], [Google Scholar]
- Albasher G, Bin-Jumah M, Alfarraj S. Finasteride and suicide: A postmarketing case series. Biosci Rep. 2020;40:BSR20191434. [Crossref], [Google Scholar], [Indexed]
- Hirshburg JM, Kelsey PA, Therrien CA, Gavino AC, Reichenberg JS. Adverse effects and safety of 5-alpha reductase inhibitors (finasteride, dutasteride): A systematic review. J Clin Aesthet Dermatol. 2016;9:56-62. [Crossref], [Google Scholar], [Indexed]
- Traish AM, Hassani J, Guay AT, Zitzmann M, Hansen ML. Adverse side effects of 5α-reductase inhibitors therapy: Persistent diminished libido and erectile dysfunction and depression in a subset of patients. J Sex Med. 2011;8:872-84. [Crossref], [Google Scholar], [Indexed]
- Giatti S, Diviccaro S, Panzica G, Melcangi RC. Post-finasteride syndrome and post-SSRI sexual dysfunction: Two sides of the same coin?. 2018;61:180-93. [Crossref], [Google Scholar], [Indexed]
- Traish AM. Post-finasteride syndrome: A surmountable challenge for clinicians. Fertil Steril. 2020;113:21-50. [Crossref], [Google Scholar], [Indexed]
- Chiriacò G, Cauci S, Mazzon G, Trombetta C. An observational retrospective evaluation of 79 young men with long-term adverse effects after use of finasteride against androgenetic alopecia. Andrology. 2016;4:245-50. [Crossref], [Google Scholar], [Indexed]
- Rezende HD, Dias MFRG, Trüeb RM. A comment on the post-finasteride syndrome. Int J Trichology. 2018;10:255-61. [Crossref], [Google Scholar], [Indexed]
- Ganzer CA, Jacobs AR. Emotional consequences of finasteride: Fool's gold. Am J Mens Health. 2018;12:90-95. [Crossref], [Google Scholar], [Indexed]
- Walf AA, Kaurejo S, Frye CA. Research brief: Self-reports of a constellation of persistent antiandrogenic, estrogenic, physical, and psychological effects of finasteride usage among men. Am J Mens Health. 2018;12:900-906. [Crossref], [Google Scholar], [Indexed]
- Birzniece V, Bäckström T, Johansson IM, Lindblad C, Lundgren P, Löfgren M, et al. Neuroactive steroid effects on cognitive functions with a focus on the serotonin and GABA systems. Brain Res Rev. 2006;51:212-39. [Crossref], [Google Scholar], [Indexed]
- Traish AM. The Impact of the 5α-reductase inhibitors (5α-ris) on male sexual function and psychological well-being. Curr Sex Health Rep. 2015;7:210-219. [Crossref], [Google Scholar]
- Römer B, Gass P. Finasteride-induced depression: New insights into possible pathomechanisms. J Cosmet Dermatol. 2010;9:331-2. [Crossref], [Google Scholar], [Indexed]
- Fertig R, Shapiro J, Bergfeld W, Tosti A. Investigation of the plausibility of 5-alpha-reductase inhibitor syndrome. Skin Appendage Disord. 2017;2:120-129. [Crossref], [Google Scholar], [Indexed]
- Romer B, Pfeiffer N, Lewicka S. Finasteride treatment inhibits adult hippocampal neurogenesis in male mice. Pharmacopsychiatry. 2010;43:174-178. [Crossref], [Google Scholar], [Indexed]
- Nguyen DD, Marchese M, Cone EB, Paciotti M, Basaria S, Bhojani N, et al. Investigation of suicidality and psychological adverse events in patients treated with finasteride. JAMA Dermatol. 2021;157:35-42. [Crossref], [Google Scholar], [Indexed]
- Welk B, McArthur E, Ordon M, Anderson KK, Hayward J, Dixon. Association of suicidality and depression with 5α-reductase inhibitors. JAMA Intern Med. 2017;177:683-691. [Crossref], [Google Scholar], [Indexed]
- Wakao R, Taavola H, Sandberg L, Iwasa E, Soejima S, Chandler R, et al. Data-driven identification of adverse event reporting patterns for Japan in vigi base, the WHO global database of individual case safety reports. Drug Saf. 2019;42:1487-1498. [Crossref], [Google Scholar], [Indexed]
- Rahimi AB, Pourandarjani R, Habibollahi P, Mualeki A. Finasteride induced depression: A prospective study. BMC Clin Pharmacol. 2006;6:7. [Crossref], [Google Scholar], [Indexed]




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.