Potential use of Ribosome Inactivating Proteins (RIPS) as Cancer Therapeutic and its Indigenous Sources
2 Department of Biology, Akhtar Saeed Medical and Dental College, Lahore, Pakistan
3 Department of Molecular Biology and Moleculargenetics, University of the Punjab, Lahore, Pakistan
Published: 30-Sep-2021
Citation: Maqsood Q, et al. Potential use of Ribosome Inactivating Proteins (RIPS) as Cancer Therapeutic and its Indigenous Sources. Ann Med Health Sci Res. 2021;11:20-28
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Ribosome-Inactivating Proteins (RIPs) are enzymes that have demonstrated remarkable cytotoxic activity linked to the ability of N-glycosidase activity in 28S ribosomal RNA to inactivate protein synthesis (rRNA). There are many sources of RIPs, but they are mainly found in plants, fungi, algae, and in some bacterial species. “The RIPs exactly bind with large ribosomal subunit and cleavage the specific base of adenine at 28S rRNA by N-glycosidase activity and stop protein translation”. Many factors suggest that the host could be protected from predators and viruses without losing its involvement in stress reactions or the preservation of nitrogen. Previous studies have reported the great potential of RIPs for use as cancer therapeutics. RIPs are specific in their function through targeting only cancer cells and less toxicity regarding normal cells. The main feature of RIPs used as cancer treatment is their short plasma half-life, antigenicity, and selective cytotoxicity. The pharmaceutical property of RIPs might be improved by coupling the RIPS with polyethylene glycol, which improves plasma time and decreases its antigenicity. We reviewed previous literature providing evidence about therapeutic approaches regarding RIPs due to their potential as a therapeutic application in different types of human diseases. RIPS functions as a target-specific carrier by its conjugation with toxins.
Keywords
Ribosome-inactivating protein; Immuno toxins; Conjugation; Cancer; Therapy; Apoptosis; Tumor cells
Introduction
Cancer is one of the main causes of death worldwide. Recently, it was reported that about 14 million people worldwide have been diagnosed with cancer and it is expected that this number might be increased to 22 million within the next two decades. Cancer is a multifactorial disease that can be caused by genetic or environmental factors. [1] Although the exact treatment for cancer has yet to be discovered, early detection of the disease aids in its cure. For the treatment of cancer, various drugs and therapies are used, with chemotherapy being the most commonly used. Immuno-based toxins are one of the interests in developing a drug against cancer due to their significant anti-cancer property. RIPs are novel proteins that are widely found in different types of plants and a few fungi and bacterial species. [2] RIPs are a group of cytotoxin N-glycosidases. There are three main types of RIPs based on the number of subunits and precursor sequence organization. Trichosanthin and monorcharin are the examples of type 1 RIPs with a single polypeptide chain and catalytic activity. [3] Abrin and ricin are the examples of type 2 RIPs with an active A-chain which is associated with lectin binding B-chain through a disulfide bond. The JIP60 and maize ribosome-inactivating protein are the examples of type 3 RIPs that contain a region between the protein which is removed for activation and binding of elongation factor. [4] Another function of RIPs is they inhibit different types of enzymatic activity like superoxide dismutase, depurination of DNA, RNA, poly A, and phospholipase. Different types of RIPs like gelonium and momordica have anti-HIV properties. Through the depurination of the SRL loop, cell apoptosis is induced by RIPs. Ricin is used for the treatment of leukemia. RIPs are conjugated with different types of antibodies to achieve target-specific cell apoptosis. [5]
Types of Ribosome Inactivating Proteins (RIPs)
On the base of the structure, there are three main types of RIPs. Type 1 RIPs, such as dianthins and saporins, are composed of a single polypeptide chain with a molecular weight of about 30 kDa and exhibit strong N-glycosidase activity. [6] Ricin and abrin are examples of type 2 RIPs, which are heterodimeric proteins with a size of 60 kDA-65 kDa, an enzymatic A-chain similar to type 1 RIP, and a disulfide bond to a slightly larger B-chain via lectin properties. The type 3 RIP is made up of a polypeptide chain that holds type 1 RIP in the C-terminal and N-terminal domain, but still its function is unknown. The type 3 RIPs are produced like inactive precursors which are activated through proteolytic processing events. [7-16] It is reviewed that the most powerful type 2 RIPs are stenodactylin purified from the caudex of the passifloraceae. This RIP has a very high enzyme activity in galactose-specific DNA substrates in ribosomes and herring. Stenodactyline is transported retrogradely in the central nervous system just like other RIPs purified from adenia, namely modecin and volkensin. [17]
In neuroscience, this property may have several medical and biotechnological applications for selectively lesion-specific neurons. It was reported that stenodactylin can cause multiple pathways of cell death in a neuroblastoma cell line which include primarily apoptosis but also necroptosis and free radical production. [18] Similar results have recently been achieved in acute myeloid leukemia cells, which allow stenodactyline to produce a rapid stress response, mainly due to pro-inflammatory factors and oxidative stress, and to lead to apoptosis.
For many years, RIPs, primarily monoclonal antibodies, have been studied because the therapeutic use of RIPs as toxic molecules for immunotoxins allows for the selective killing of target cells. [9] In several clinical trials with various diseases, immunotoxins were included, with promising results often achieved, in the treatment of hematological neoplasms. Because of its high potential cytotoxicity, stenodactyline could be an ideal candidate for cancer treatment and toxic movement of immunotoxins as a unique agent for local-regional treatment.
Pharmaceutical Applications of RIPs
RIPs are widely used as a part of the conjugate in different types of medicine, importantly in cancer therapy. The conjugate of RIPs is enzymatically linked with tumor-targeting ligands and antibodies which lead to targeting their binding and entry to tumor cells. As many plants are an indigenous source of RIPs, which are much more effective and cost-effective as compared to synthetic drugs. [10]
As it is reviewed the RIPs as a cytotoxin, it has been tested for many anti-tumor, antiviral, antifungal, and anti-bacterial properties. Clinical trials have been carried out for gelonin being used as a treatment of myeloid malignancies, ricin for leukemia treatment, and PAP for treatment of immunodeficiency virus. [19,20]
To make immunotoxins, the antibodies may be conjugated by RIPs which results in selective function RIPs to target cells and improve their efficacy and reduce their side effects. The polyethylene glycol might be used to couple with RIPs. The PEG and RIP complex has enlarged the molecular size, which results in renal clearance, proteolytic degradation, and decreases antigenicity and immunogenicity. [21]
Role of RIPs in Cancer Therapy
The TCS and momordica are type 1 RIPs having a protein size of 30 kDa function as anti-HIV. Gelonium is an anti-HIV protein with a size of 30 kDa. The α-MMC and marmorin inhibit the growth of breast tumour cells invivo and invitro. [22]
The cell viability is inhibited by RIPs resulting in cell cycle arrest and a decrease in the size and volume of the tumor by persuading apoptosis with caspase-8 and caspase in cells of breast tumor. To target different types of cells and solid tumors, a RIP prototype-based immunotoxin is used. The Saporin-S6 immuno conjugates have good structural and functional properties. Saporin-S6 is highly resistant to proteolysis and denaturation, has high catalytic efficacy, and is less toxic to normal cells. [19]
In many preclinical studies, Saporin-S6 has been intensively used in immune conjugates as a toxic moiety that targets different types of solid tumors and malignant hematological cells. For target therapy, the hematological cell represents the best candidate because it is easy to target invivo access as they offer surface target antigens as compared to solid tumors. [23]
The TCS facilitates the phosphoinositide 3-kinase or AKT pathway which improves apoptosis-inducing and cytotoxicity activity in anti-cancer therapy and gemcitabine against lung cancer. [24] The cell penetration of Granzyme B is enhanced by TCS, resulting in the apoptosis of tumor cells. In gastric cancer cells, the RIPs induced autophagy through a higher level of autophagy protein 5 and changing the microtubule-associated protein LC3 1 to LC3 2 which stimulates the NF-KB tumor protein p53 pathway by inducing reactive oxygen species. [15]
The extracellular Ca2+ might be responsible for the induction of reactive oxygen species which stimulate apoptosis in human choriocarcinoma cells. In JAR cells, RIPs inhibited angiogenesis by the reduction of vascular endothelial growth factor and inhibition of angiogenic signal that participated in the anti-cancer effect. The TCS also regulated the smac pathway in cervical cancer cells. [12]
It is reported that on leukemia and lymphoma, TCS shows activity against proliferation. It induces cell apoptosis of T-lymphocyte and inhibits the growth of B-lymphocyte by the arrest of S-phase of cell cycle. [25] Ricin also exhibits an anti-tumor property. Ricin inhibited the growth of sarcomas in rats, and it increased the survival rate of ehrlich ascites tumor-bearing mice. It also shows a promising effect on nude mice with human xeno graft. Riproximin is a type 2 RIP that up-regulated the anti-cancer cytokine IL24/MDA-7 and ER-stress-related GADD genes: It also down-regulated the genes relating to migration (Rho GTPases), anti-apoptotic activities (BCL family), and cell cycle (cyclins) in selected human breast cancer cells MDA-MB-231 and MCF-7. [26]
Α-MMC might be used for the treatment of macrophages associated with the tumor. The cytokine release is regulated by α-MMC. Curcin is a type 1 RIP that stops the growth of different types of tumor cell lines. T-cell leukemia could be selectively inhibited by viscumarticulatum RIP. And the early signalling of articulatin D induces apoptosis by increasing the level of mitochondrial membrane potential. “RTA conjugated with anti-HER2 scFv 4D5 and the endoplasmic reticulum-targeting peptide KDEL had a 440-fold increase in anti-ovarian cancer cell activity compared to RTA alone”. [27]
RIPs Mechanism of Action
Typically, RIP enters the cell by binding to a receptor on the cell's surface, and then through endocytosis, RIPs cross the cell membrane and translocate into the cytosol. Through endocytosis, the type 1 RIP across the cell membrane through its binding with galactose moieties and vesicular transport it transfers from the network to the endoplasmic reticulum. [28] The portion of chain-A transfers to the cytoplasm when the A and B chains of ER tumen are dissociated. To build a target bifunctional cytotoxic agent, the membranes of RIP might be fused or conjugated with suitable carriers like hormones, protease, inhibitors, and cell-binding ligands. [29,30] It has been reported in various studies that a transferrin conjugated carrier design is used, which is a type of protein through which iron uptake occurs. Iron-binding glycoprotein transferrin carries two irons into the cell in the form of ferric Fe3+ when it binds to its receptors. [31]
The transferrin receptor is extensively distributed among different types of cells but is commonly overexpressed in malignant cells. For example, Saproin-S6, ricin is a type of artificial conjugate. [32] These conjugates are artificially formed by the combination of RIP and transferrin that involves different types of mechanisms like selective cytotoxicity on many malignant or cancerous cell lines. [33]
Through RNA N-glycosides activity, the RIPs enter the cell and inactivate the eukaryotic ribosome. The glycoproteins or glycolipids are present on the cell membrane, and the type 2 RIP binds to them. After binding, the RIP travels through an intracellular pathway from the golgi apparatus to the endoplasmic reticulum. [34] Unless the RIPs exploit the ER-associated degradation pathway, the enzymatic moieties are not released to the cytosol and will not reach the ribosome to exert their function. Because the type 1 RIP is difficult to bind with sugar-binding receptors, it is tough for it to enter the cell, because the type 1 RIP interacts with phospholipids on the cell membrane, it enters the cell through this binding interaction. The type 1 RIP is linked with a carrier like monoclonal antibodies to facilitate its entry into the cell and these conjugates might be toxic to target cells. [35]
Cellular mechanism of RIPs
Mechanism of entry in the cell: Ribosome-inactivating proteins must enter cells to inactivate the eukaryotic ribosome via their RNA N-glycosidase activity. It is difficult for type 1 RIPs to enter cells because of their sugar-binding activity deficiency. The exact entry mechanism remains unclear. [36]
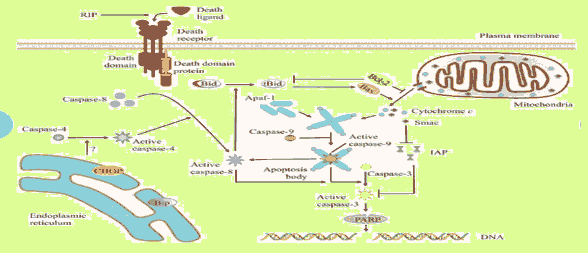
Mitochondria apoptosis mediated by RIPs: Apoptosis-inducing substances can lead to excessive reactive oxygen species production. Mitochondrial membrane potential depolarization and caspase-9 activation were detected in MCF-7 cells and to a lesser extent in MDA-MB-231 cells after marmorean treatment. Several pumps, such as the Na+–K++ pump and the Ca++ pump, maintain concentration gradients of various ions to achieve appropriate membrane potential. [37]
Death receptor apoptosis mediated by RIPs: Death receptors such as fas, deliver apoptotic signals into the cytoplasm by binding to Fas Ligand (FasL). Marmorin was found to trigger the death receptor apoptotic pathway in MCF7 cells. This pathway is also preferentially activated in MDA-MB-231 cells. [38]
RIP-Based Immunotoxins
The combined antibody and specific toxins obtained by the molecules are known as immunotoxins, bifunctional macromolecular based on intracellular toxin action to kill target cells. Recombining antibody technology and protein fusion has led to the rapid growth of drug targeting systems with higher antigen-binding characteristics and pharmacokinetic characteristics. [38,39] In immune regulation and treatment of viral or parasitary illnesses, immunotoxins are currently regarded as strong immune bubbles against cancer cells. Previous studies show that anti conjugation methods can theoretically influence immune conjugate activity in vivo. Different critical strategies were implemented to maximize active toxin releases in the target cell. [8] The choice of reduced disulfide cross-linker between RIP mode and antibodies is one of most RIP-based immune conjugate synthesis approaches for tumor cells, which are typically characterized by a hypoxic condition, induced by increased reductive enzyme activity. Redox target cell status should therefore be able to induce RIP-based immune conjugate decrease after cell internalization with the resulting discharge of type 2 catalytic active A chain or type 1 RIP chain. [13] An alternative approach for RIPs without free gum sulphide (e.g. gelonine, PAP, saporin) and also used for whole type 2 RIPs, was based on the application of a specific linker binding reactive functional groups that generate new sulphydrylic acids on the proteins, such as primal amines, carbonyls, carbohydrates, and carbonyl acids. Preparation of a separate derivative of both toxins and antibodies was required for this strategy; newly inserted groups on two molecules can react to a stable conjugate population via disulfide bridges. [40] SPDP (N-Succinamidyl-3-(2-Pyridyldithio) Propionate); SATA (S-(N-Succinimidyl) Thioacetate); SMPT ((N-Succinimidyloxy carbonyl)-1-Methyl-1-(2-Pyridyldithio) Toluene) are some of the most common hetero bifunctional cross-linking agents in the immunotoxins conjugation of S-S disulfide.
Use of RIPs as Cancer Therapeutics
In 1987, Griffiths reported that RIPs can induce cell death. Through this evidence, it is concluded that one of the pathways persuaded by RIPs in which RNA N-glycosidase activity might be responsible for ribotoxin stress which results in the activation of p38 MAPK and SAPK that leads to apoptosis. However, the mechanism of signal transmission from damaged 28S rRNA to the MAPK remains to be investigated further. [41]
The extraordinarily high level of potency is a common characteristic of many RIPs. For example, diphtheria is another protein synthesis inhibiting toxin. It is supposed that a small number of molecules are needed to enter the cytosol of the cell, leading to cell death. [42] This effectiveness of RIPs makes them the most attractive for use as cancer therapeutics. Moreover, to convey specific anti-cancer activity, the toxin must target specific means it targets only malignant cells, not healthy cells. The main method through which the target-specific anti-cancer activity of RIPs is achieved through its conjugation of RIPs enters with antibody to make immunotoxin or to target ligand-like cytokine or growth factor. The use of immunotoxins is the most suitable choice because they allow flexibility and selectivity to target-specific choices. The selection of a suitable target is very important because it has large effects on its potency and specificity. Because of these constraints on tumor toxicity targets, a target that is highly expressed on the surface of cancer cells but limited in its expression on normal cells must be chosen. [43]
The immunotoxin generally targets the tumor-linked antigen that is highly expressive on the surface of the cell due to transformation. In the 1970s, the first generation of immunotoxins was made considering RIPs alone, more than 450 immunotoxins have been used. Mostly plant-based RIPs immunotoxins were developed. [39] Ricin is derived from gelonin and saporin two types 1 RIPs with high potency and stability, via chain A of type 2 RIP ricin. Recently, it has been suggested that the type 2 RIPs are used with low in vivo and in vitro toxicity, but in the free cell, the system shows strong ribosomal RNA N-glycosidase activity. The chain A of nigrin and ebulins, like in sambucus species, is used to generate alternative immunotoxins with increased cytotoxicity. As recombinant technology has advanced, instead of making chemical-based conjugates, an active toxin is genetically engineered with whole antibody and domains are fused by human antibody fragments. This process of development of immunotoxins is divided into three generations. [14]
RIPs Effects on Breast Cancer
RIPs TCS, momordica anti-Human Immunodeficiency Virus (HIV) protein of 30 kDa (MAP30), gelonium anti-HIV protein of 31 kDa (GAP31), gelonin, marmorin, and alpha-momorcharin (α-MMC) have been shown to negatively affect the growth of breast tumor cells in vivo and in vitro. MAP30 and GAP31 could represent a therapeutic strategy for the treatment of breast cancer. The combination could decrease HER2 expression and lead to reduced Fn14 expression, which is frequently co-expressed in human breast tumors. [44] Ribosome-inactivating proteins have the potential to become innovative anti-tumor agents. They also possess toxic adverse effects, including severe systemic anaphylaxis, immunogenicity, and toxicity to reduce the undesirable effects and achieve better therapeutic efficacy [Table 1].
| Table 1: Different indigenous sources of RIPs. | |||
|---|---|---|---|
| Sr No. | RIPs type | Source | Reference |
| 1 | Ricinus communis | Castor oil | 4 |
| 2 | Type I RIPs | Maize and barley | 5 |
| 3 | Ricinus agglutinin | Castor beans | 6 |
| 4 | Ricin type 2 RIP | Sambucus species | 7,8 |
| 5 | Type1 and type 2 RIP | Annona squamosa | 9,10 |
| 6 | Ricin a type 2 RIP | Ricinus communis | 9 |
| 7 | TCS a type 1 RIP | Sambucus ebulus | 8 |
| 8 | Heterodimeric type II RIPs | Rhizome | 11 |
| 9 | Curcin, a broad cytotoxic type 1 RIP | Seeds of Jatropa curcas L | 12 |
| 10 | Sporins a type 1 RIPs | Seeds of Triticum aestivum L | 12 |
| 11 | Type 1 Ribosome-Inactivating Protein (RIP) saporin-S6 | Saponaria officinalis L. Seeds | 13,14 |
| 12 | Foetidissimin II | Cucurbita foetidissima | |
| 13 | Type 1 RIP B. Texanin | Cucurbita texana | 14 |
| 14 | Mayahuelin type 1 RIP | Roots of Cucurbita foetidissima | 15 |
| 15 | Abrin type 2 RIP | Fruits of Cucurbita texana | 16 |
| 16 | Type 1 RIPs like dianthins | Barley and Jatropha curcas | 16 |
RIPs effects on leukemia and lymphoma
Trichosanthin significantly inhibits the proliferation of various leukemia and lymphoma cell lines. Cucurmosin and TCS down-regulate P210Bcr-Ab1 and inhibit tyrosine kinase, resulting in cell growth suppression. [45] Saporin and rgel have been widely used to construct immunotoxins, which have been reported to be useful in cancer treatment. HB22.7-saporin was cytotoxic against a panel of non-Hodgkin's lymphoma (NHL) cell lines and was shown to significantly prevent tumor development in a xenograft model of NHL. [46]
RIPs effects on other cancers
Recently, MAP30 has been demonstrated in cell culture and mice for antitumor activity. In HepG2 cells, for example, time and dosage-dependent cell viability with S Phase, arrest were inhibited by MAP30. Furthermore, MAP30-induced apoptosis and necrosis led to a decrease of tumor volume in HepG2-bearing mice. Cucurmosin caused the arrest and apoptosis of G0/G1 in HepG2 cells, also translating these effects to powerful anti-tumor activity in vivo. In HepG2 cells, abrus agglutinin activates the cascade while suppressing act phosphorylation and NF-B expression. [11]
Engineering of RIPs for Improving Anti-Cancer Activity
RIPs ITs show high anti-cancer activity mainly for hematological malignancies that are easier to access than solid tumors. The clusters of differentiation present on the surface of hematological cells are considered the most appropriate target to design better ITs. The antigens CD7, CD2, Cd19, and CD22, present on the surface of human lymphoma and leukemia were linked with Sap-So6 to make its. [47-51] The selective cytotoxicity of saporin ITs increased 100-fold more than the use of Saporin alone. It was reported that anti-CD30-Saporin decreased 60% of tumor mass when it was used for the treatment of refractory Hodgkin lymphoma patients. Previous studies show that anticonjugation methods can theoretically influence immunoconjugate activity in vivo. Different critical strategies were implemented to maximize active toxin releases in the target cell. [8] The choice of reduced disulfide cross-linker between RIP mode and antibodies is one of most RIP-based immune conjugate synthesis approaches for tumor cells, which are typically characterized by a hypoxic condition, induced by increased reductive enzyme activity. Redox target cell status should therefore be able to induce RIP-based immune conjugate decrease after cell internalization with the resulting discharge of type 2 catalytic active A chain or type 1 RIP chain. [13] An alternative approach for RIPs without free gum sulphide (e.g. gelonine, PAP, saporin) and also used for whole type 2 RIPs, was based on the application of a specific linker binding reactive functional groups that generate new sulphydrylic acids on the proteins, such as primal amines, carbonyls, carbohydrates, and carbonyl acids. Preparation of a separate derivative of both toxins and antibodies was required for this strategy; newly inserted groups on two molecules can react to a stable conjugate population via disulfide bridges. [40] SPDP (N-Succinamidyl-3-(2-Pyridyldithio) Propionate); SATA (S-(N-Succinimidyl) Thioacetate); SMPT ((N-Succinimidyloxy carbonyl)-1-Methyl-1-(2-Pyridyldithio) Toluene) are some of the most common heterobifunctional cross-linking agents in the immunotoxins conjugation of S-S disulfide [Figures 1-5].
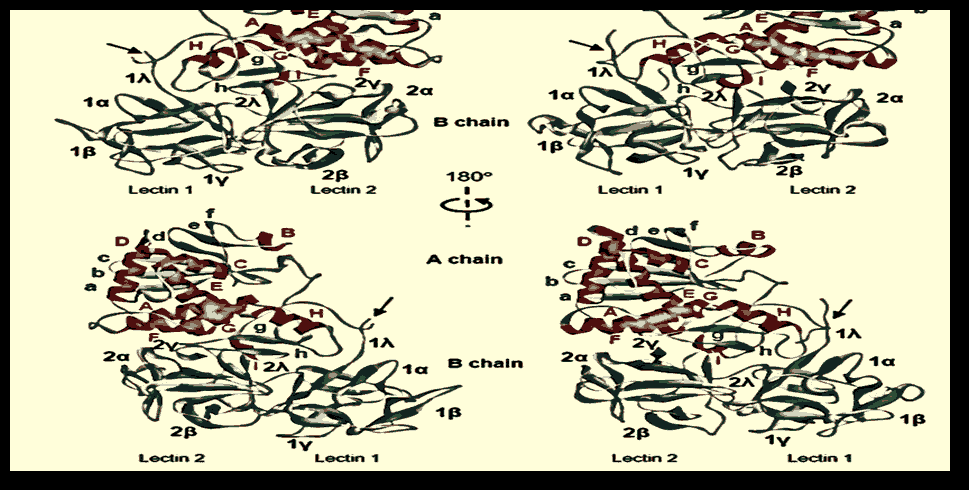
Figure 1: Compared to ricin, the structure of stenodactylin. A) The A-B-stenodactyl and ricin amino acid sequence alignment. The β-strands (blue), α-helices (red), and the disulfide-binding cysteines are shown (in yellow). The helixes are tagged A to I and the β-sheet strands are tagged A to h in the A chain. Also indicated are the domains and subdomains in the B chain. Similar resides, conserved replacements, and semi-conserved replacements are reported. The various structural motifs in the chains A and B. A) Stenodactylin's three-dimensional compared to ricin structure. The α helices (red) are represented, the α chains (cyan) and the spindles (grey). The helices are A to I marketed and the β sheet strands are A to H marked. In the B chain are shown structural areas and subdomains. Arrows indicate the disulfide bond position between chains A and B.
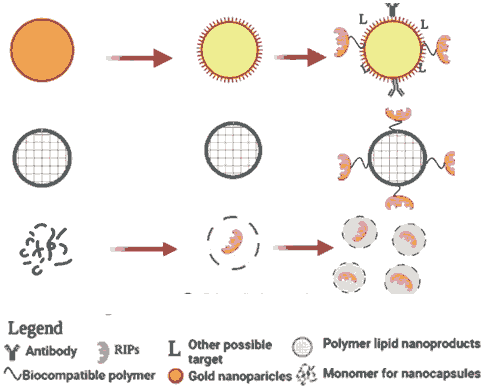
Figure 5: Potential use of a toxic plant RIP (green star and 3D-ribbon) beginning with the classic IgG immunotoxin chemically conjugated to a native RIP that may be further transformed (left) into a re-fusion or (right) into a single chain variable fusion into a RIP or even simply into DNA, encoding the plant RIP with all the different molecular components. Targeted exosomes or engineered nanoparticles with MnFe2O4 nanoparticles, even for diagnostic purposes.
Conclusion
Through the review of previous studies, it is concluded that RIPs are cytotoxic plant proteins with higher anti-cancer, anti-HIV, and anti-viral properties. Based on the structure, there are three main types of RIPs. Moreover, it has limitations like other therapeutic agents, for example, immunogenicity, non-specificity, fast degradation, etc. In the same plant, single-chained type 1 RIPs can coexist with double-chained type 2 RIPs. Small RIPs, such as alpha-momorcharin, beta-momorcharin, gamma momorcharin, and charantin in bitter gourd (charantia) seeds, alpha- and beta-kirilowins, may coexist with larger RIPs in the same plant. Today's pharmacology is focused not only on the discovery of “nano-compounds”, but also on the development of the second line of bio-based chemotherapeutic conjugates, through utilizing both recombinant protein engineering and protein technology. For this reason, the pharmaceutical and pharmacology businesses are examining a variety of options, including specific cellular targets and novel drug delivery techniques. Because of their topology and toxicity, RIPs proved to be good prototypes in both scenarios. A nanotechnology-based delivery system might be helpful for the development of anti-cancer drugs. Several drugs have been nanocarriers and they are highly effective for therapeutic use. There are many indigenous sources of RIPs, mainly plants. It is easy to isolate RIPs from plants and is more productive and cost-effective. However, it needed to further investigate the potential of RIPs as cancer therapeutics.
Reference
- Ajji PK, Sonkar SP, Walder K, Puri M. Purification and functional characterization of recombinant balsamin, a ribosome-inactivating protein from momordica balsamina. Int J Biol Macromol. 2018;114:226-234.
- Ali A, Zaman A, Sayed E, Evans D, Morgan S, Samwell C, et al. Electro hydrodynamic atomisation driven design and engineering of opportunistic particulate systems for applications in drug delivery, therapeutics and pharmaceutics. Adv Drug Deliv Rev. 2021;176.
- Asrorov AM, Gu Z, Min KA, Shin MC, Huang Y. Advances on tumor-targeting delivery of cytotoxic proteins. ACS Pharmacol. Transl. Sci. 2019;3:107-118.
- Barbier J, Gillet D. Ribosome inactivating proteins: From plant defense to treatments against human misuse or diseases. MDPI. 2018.
- Bolognesi A, Bortolotti M, Battelli MG, Polito L. Hyperuricaemia, xanthine oxidoreductase and ribosome‐inactivating proteins from plants: the contributions of fiorenzo stirpe to frontline research. mol. 2017;22:206.
- Bortolotti M, Maiello S, Ferreras JM, Iglesias R, Polito L, Bolognesi A. Kirkiin: A new toxic type 2 ribosome-inactivating protein from the caudex of adenia kirkii. Toxins. 2021;13:81.
- Bulgari D, Landi N, Ragucci S, Faoro F, Di Maro A. Antiviral activity of PD-L1 and PD-L4, type 1 ribosome inactivating proteins from leaves of phytolacca dioica l, In the pathosystem phaseolus vulgaris-Tobacco Necrosis Virus (TNV). Toxins. 2020;12:524.
- Rezaei MM, Bandehagh A, Dehestani A, Pakdin PA, Golkar M. Molecular cloning and in-depth bioinformatics analysis of type II ribosome-inactivating protein isolated from Sambucus ebulus. Saudi J Biol Sci. 2020;27:1609-23.
- Chen YJ, Zhu JQ, Fu XQ, Su T, Li T, Guo H, et al. Ribosome-inactivating protein α-momorcharin derived from edible plant momordica charantia induces inflammatory responses by activating the NF-kappa B and JNK pathways. Toxins. 2019;11:694.
- Dhanraj SR, Vennila JJ, Dhanraj M. Pharmacological investigation of Ribosome Inactivating Protein (RIP)-like protein extracted from annona squamosa L. J King Saud Univ Sci. 2020;32:2982-8.
- Weise C, Schrot A, Wuerger LT, Adolf J, Gilabert OR, Sama S, et al. An unusual type I ribosome-inactivating protein from agrostemma githago L. Sci Rep. 2020;10:1-11.
- Kokorin A, Weise C, Sama S, Weng A. A new type 1 ribosome-inactivating protein from the seeds of gypsophila elegans M. Bieb. Phytochemistry. 2019;157:121-7.
- Rezaei MM, Dehestani A, Bandehagh A, Pakdin PA, Golkar M, Heidari JR. Recombinant pebulin protein, a type 2 ribosome-inactivating protein isolated from dwarf elder (Sambucus ebulus L.) shows anticancer and antifungal activities in vitro. Int J Biol Macromol. 2021;174:352-61.
- Soria MR, Benatti L, Nitti G, Ceriotti A, Solinas M, Lappi DA, et al. Studies on ribosome-inactivating proteins from saponaria officinalis. Genetically Engineered Toxins: CRC Press. 2020.193-212.
- Zhu F, Zhou YK, Ji ZL, Chen XR. The plant ribosome-inactivating proteins play important roles in defense against pathogens and insect pest attacks. Front Plant Sci. 2018;9:146.
- Zhang Y, Yang Q, Li C, Ding M, Lv X, Tao C, et al. Curcin C, a novel type I ribosome-inactivating protein from the post-germinating cotyledons of jatropha curcas. Amino acids. 2017;49:1619-31.
- Chandran T, Vijayan M, Sharma A. Structural studies on non-toxic homologues of type ii Ribosome Inactivating Proteins (RIPS). Journal of Bioscience. 2019;40.
- Chen Y, Yu Z, Tse A. Inflammatory properties of ribosome-inactivating protein momorcharin derived from bitter melon: Abridged secondary publication. Hong Kong Med J. 2021;27.
- Girardi T, Vereecke S, Sulima SO, Khan Y, Fancello L, Briggs JW, et al. The T-cell leukemia-associated ribosomal RPL10 R98S mutation enhances JAK-STAT signaling. Leukemia. 2018;32:809-19.
- Fabbrini MS, Katayama M, Nakase I, Vago R. Plant ribosome-inactivating proteins: Progesses, challenges and biotechnological applications (and a few digressions). Toxins. 2017;9:314.
- Fan X, Wang Y, Guo F, Zhang Y, Jin T. Atomic-resolution structures of type I ribosome inactivating protein alpha-momorcharin with different substrate analogs. Int J Biol Macromol. 2020;164:265-76.
- Ghosh T, Vashi Y, Barman K, Singha L. Novel Ribosome-inactivating Protein (RIP) isolated from trichosanthes dioica induces apoptosis in hela cell line. Nat Resour J. 2021.
- Iglesias R, Citores L, Ferreras JM. Ribosomal RNA N-glycosylase activity assay of ribosome-inactivating proteins. Bio Protocol. 2017;7:e2180.
- Ismail H, Ciptasari UH, Ikhsan MAN, Suryani F, Sismindari S, Martien R, et al. Conjugation of anti-EpCAM antibody on alginate–RIP MJ-30 nanoparticle through carbodiimide reaction as a model of targeted protein therapy. Indones J Pharm. 2019;30:52.
- Landi N, Ruocco MR, Ragucci S, Aliotta F, Nasso R, Pedone PV, et al. Quinoa as source of type 1 ribosome inactivating proteins: A novel knowledge for a revision of its consumption. Food Chem. 2021;342:128337.
- Lestariana WLW. Identification and test of active protein resemble Ribosomr-Inactivating Proteins (RIPs) on kaemferia rotunda linn. J Med Sci. 2020;35.
- Lin Y, Xu L, Li Y, Wu X, Liu Y, Zhu H, et al. Ribosome-Inactivating Proteins (RIPs) of bougainvillea glabra uncovered polymorphism and active site divergence. Toxins. 2021;13:331.
- Liu WY. Research on ribosome-inactivating proteins from angiospermae to gymnospermae and cryptogamia. Am J Transl Res. 2017;9:5719.
- LiuY, Cao XW, Lu MY, Wang FJ, Zhao J. Enhancement of anti-tumor effect of a ribosome-inactivating protein by cell penetrating peptides and saponin. Biotechnol Bull. 2019;35:146.
- Wensley HJ. Mechanism of triterpenoid saponin mediated augmentation of saporin based immunotoxin cytotoxicity. University of Southampton. 2019.
- Lu JQ, Zhu ZN, Zheng YT, Shaw PC. Engineering of ribosome-inactivating proteins for improving pharmacological properties. Toxins. 2020;12:167.
- Lu W, Mao Y, Chen X, Ni J, Zhang R, Wang Y, et al. Fordin: A novel type I ribosome inactivating protein from Vernicia fordii modulates multiple signaling cascades leading to anti-invasive and pro-apoptotic effects in cancer cells in vitro. Int J Oncol. 2018;53:1027-42.
- Maiello S. Characterization and cytotoxic mechanisms of toxic and non-toxic type 2 ribosome-inactivating proteins. Alma DL University of Bologna Digital Library. 2017.
- Mercatelli D, Bortolotti M, Giorgi FM. Transcriptional network inference and master regulator analysis of the response to ribosome-inactivating proteins in leukemia cells. Toxicology. 2020;441:152531.
- Mudgal G. Potentials of investigations on mistletoe ribosome inactivating proteins. Plant Cell Biotechnol Mol Biol. 2020:158-68.
- Murtaja A, Eyol E, Xiaoqi J, Berger MR, Adwan H. The ribosome inhibiting protein riproximin shows antineoplastic activity in experimental pancreatic cancer liver metastasis. Oncol Lett. 2018;15:1441-1448.
- Pertiwi D, Martien R, Ismail H. Formulation of nanoparticles Ribosome Inactivating Proteins from Mirabilis Jalapa L.(RIP MJ) conjugated AntiEpCAM antibody using low chain chitosan-pectin and cytotoxic activity against breast cancer cell line. Pak J Pharm Sci. 2018;31.
- Polito L, Bortolotti M, Iglesias R, Bolognesi A. Toxic plant proteins as experimental drugs for human pathologies. Front pharmacol. 2021;12.
- Shin MC, Min KA, Cheong H, Moon C, Huang Y, He H, et al. Tandem-multimeric F3-gelonin fusion toxins for enhanced anti-cancer activity for prostate cancer treatment. Int J Pharm. 2017;524:101-10.
- Roy S, Axup JY, Forsyth JS, Goswami RK, Hutchins BM, Bajuri KM, et al. SMI-ribosome inactivating protein conjugates selectively inhibit tumor cell growth. Chem Comm. 2017;53:4234-7.
- Rust A, Partridge LJ, Davletov B, Hautbergue GM. The use of plant-derived ribosome inactivating proteins in immunotoxin development: Past, present and future generations. Toxins. 2017;9:344.
- Setayesh MZ, Poorsargol M. Toxic proteins application in cancer therapy. Mol Biol Rep. 2021:1-14.
- Shi WW, Wong KB, Shaw PC. Structural and functional investigation and pharmacological mechanism of trichosanthin, a type 1 ribosome-inactivating protein. Toxins. 2018;10:335.
- Van DEJ. Ribosome-Inactivating Proteins (RIPs)-commemorative issue in honor of professor fiorenzo stirpe. MDPI. 2017.
- Zhou Y, Li XP, Kahn JN, Tumer NE. Functional assays for measuring the catalytic activity of ribosome inactivating proteins. Toxins. 2018;10:240.
- Wani SS, Dar PA, Zargar SM, Dar TA. Therapeutic potential of medicinal plant proteins: present status and future perspectives. Curr Protein Pept Sci. 2020;21:443-87.
- Deasy P, Ronny M, Hilda I. Formulation of nanoparticles Ribosome Inactivating Proteins from Mirabilis Jalapa L.(RIP MJ) conjugated AntiEpCAM antibody using low chain chitosan-pectin and cytotoxic activity against breast cancer cell line. Pak J Pharm Sci. 2018;31:379-384.
- Weng A. A novel adenine-releasing assay for ribosome-inactivating proteins. J Chromatogr B. 2018;1072:300-304.
- Wong JH, Bao H, Ng TB, Chan HHL, Ng CCW, Man GCW, et al. New ribosome-inactivating proteins and other proteins with protein synthesis–inhibiting activities. Appl Microbiol Biotechnol. 2020;104:4211-26.
- Yaqoob R, Noureen A, Ujjan JA, Achakzai WM, Saddozai S, Saleem N, et al. Functional importance and anti-tumor activities of Ribosome Inactivation Protein (RIPs). Ann Romanian Soc Cell Biol. 2021; 25:18747-63.
- Zaeytijd JD, Van Damme EJ. Extensive evolution of cereal ribosome-inactivating proteins translates into unique structural features, activation mechanisms, and physiological roles. Toxins. 2017;9:123.






 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.