Prevalence of Sleep Impairment and Assessment of Sleep Patterns using Pittsburgh Sleep Quality Index in Patients with Chronic Obstructive Pulmonary Disease
Citation: Adetiloye AO, et al. Prevalence of Sleep Impairment and Assessment of Sleep Patterns using Pittsburgh Sleep Quality Index in Patients with Chronic Obstructive Pulmonary Disease. Ann Med Health Sci Res. 2018;8:304-309
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Background: Impaired sleep is reportedly common in chronic obstructive pulmonary disease (COPD) and its impact on Health-related quality of life has been documented. However, there is scarcity of data on sleep patterns and quality among patients with COPD in Nigeria. Methodology: Sixty clinically stable patients with COPD were evaluated. Information on demographics and presence of nocturnal symptoms were obtained. The Modified Medical Research Council (MMRC) dyspnea scale was used to assess severity of dyspnea. Subjective sleep quality was assessed using the Pittsburgh Sleep Quality Index (PSQI) and Daytime sleepiness was assessed using the Epworth sleepiness scale (ESS). Additionally, lung function was assessed by spirometry. In all the statistical tests, a p value of <0.05 was considered significant. Results: The mean age of the study population was 70 ± 8 years. Forty-nine patients (81.7%) had poor quality of sleep (PSQI >5), with sleep duration and habitual sleep efficiency sub-scores contributing the most to the total PSQI score. Three patients (5%) had excessive daytime sleepiness (ESS>9). Fortyfour (73.3%) patients reported disease related symptoms at night; cough (36.7%) was the most common nigh-time symptom. Severity of dyspnea and COPD according to Global Initiative for Obstructive Lung Disease (GOLD) stage were significantly associated with sleep quality (p=0.01 and 0.003 respectively). Conclusion: Results from this study suggest that overall sleep quality is poor in patients with COPD with many experiencing nighttime symptoms. However, excessive daytime sleepiness was not common among the study population.
Keywords
Chronic Obstructive Pulmonary Disease (COPD); Pittsburgh Sleep Quality Index (PSQI)
Introduction
COPD is a major cause of chronic morbidity and mortality throughout the world. It represents an important public health challenge that is both preventable and treatable. [1] According to a recent report, COPD is characterized by persistent airflow limitation that is usually progressive and is enhanced by chronic inflammatory response in the airways and lungs to noxious particles and gases. [1]
It is the fourth leading cause of death in the world. [1] At least 65 million people worldwide have severe COPD. [2] COPD is also an important cause of morbidity in the middle to elderly population in Nigeria with 10.5% having GOLD stage 2 and higher COPD. [3] COPD is associated with several respiratory and non-respiratory symptoms. Among the non-respiratory symptoms are weight losses, anxiety and sleep disturbances. [1] Sleep is a complex reversible state characterized by diminished responsiveness to external stimuli and a universal need of all higher life form, absence of which has serious physiological consequences. [4,5]
Sleep disturbances are particularly prevalent in COPD with associated poor outcomes. [6-8] Its aetiology is multifactorial and include demographic factors, pharmacotherapy, night-time diseasespecific symptoms, nocturnal hypoxemia, presence of comorbid sleep disorders, anxiety and depression as well as other medical conditions. [7] The nature of sleep problems among Nigerian patients with COPD has been poorly characterized, and studies on the clinical consequences of sleep disturbance in COPD are limited. [7]
Methodology
This study is a cross sectional analytical study. Sixty subjects with diagnosis of COPD according to GOLD guidelines were consecutively recruited for the study. Inclusion Criteria are Patients with COPD diagnosis as defined by the Global initiative for chronic Obstructive Lung Disease (GOLD) guideline that are clinically stable. Shift workers and patients with medical conditions that may contribute to dyspnoea and exercise limitations such as corpulmonale and congestive heart failure were excluded. A modified version of the Medical Research Council (MRC) questionnaire was used to obtain information on respiratory symptoms of the patients. [8] Other parameters collected at the time of enrollment included age, sex, body mass index and most disturbing night-time symptoms in the previous month.
Sleep quality
Sleep quality was assessed using the Pittsburgh sleep quality index (PSQI), a self-rating scale designed to measure perceived quality of sleep. It differentiates “poor” from “good” sleep by measuring seven domains: subjective sleep quality, sleep latency, sleep duration, sleep efficiency, sleep disturbances, use of sleep medication, and daytime dysfunction over the last month. The seven domains sub scores each of which ranging from 0 to 3 are tallied, yielding a “global” score that range from 0 to 21 with increasing score meaning worse sleep quality. A global score of >5 is accepted as differentiating good from poor sleepers with diagnostic sensitivity and specificity of 89.6% and 86.5% respectively. [9]
Pulmonary function
Pulmonary function test was performed according to the American thoracic society guideline (ATS) using a standardized spirometer. The following parameters were measured: Forced Expiratory Volume in 1second (FEV1), Forced Vital Capacity (FVC) and FEV1/FVC ratio. Subjects were divided into four groups on the basis of the severity of their airway obstruction using the GOLD criteria. [1]
Data analysis
Frequency tables and charts were used to describe the age distribution, gender, demographic and clinical characteristics of the study population. One-way ANOVA and Independent-Samples T-Test were used to compare mean values. In all the statistical tests, a p value of <0.05 was considered significant.
Results
The mean age of the patients was 70 ± 8 years with the age range between 50 and 87 years. About a third of the patients gave a positive history of cigarette smoking (20, 33.3%) while 3 (5%) patients were current smokers. The mean pack years for current smokers are 11.5 ± 9.1 years while ex-smokers had a mean pack year of 8.9 ± 11.5 years. In the majority of the study subjects, the use of firewood is the source of cooking fuel (42; 70.0%). Other baseline characteristics and mean PSQI scores are shown in Table 1.
| Baseline characteristics | Number of patients N= 60 | Percentage (%) | Global PSQI score Mean (SD) | p-value |
|---|---|---|---|---|
| Age distribution | ||||
| 50-59 | 8 | 13.3 | 9.4 (5.6) | 0.571 |
| 60-69 | 12 | 20.0 | 9.1 (3.5) | |
| 70-79 | 31 | 51.7 | 10.7 (4.3) | |
| =80 | 9 | 15.0 | 9.9 ± 3.4 | |
| Gender Distribution | ||||
| Male | 32 | 83.3 | 9.94 (4.2) | 0.514 |
| Female | 28 | 46.7 | 9.93 (4.3) | |
| Body mass index | ||||
| <18.5 | 14 | 23.3 | 10.3 (4.2) | 0.681 |
| 18.5-24.9 | 33 | 55.0 | 9.5 (3.9) | |
| 25-29.9 | 11 | 18.3 | 11.1 (5.4) | |
| =30 | 2 | 3.3 | 8.5 ± 2.1 | |
| Educational status | ||||
| No formal education | 27 | 45.0 | 11.0 (4.3) | 0.023 |
| Primary education | 14 | 23.3 | 8.6 (3.0) | |
| Secondary education | 12 | 20 | 11.3 (4.5) | |
| Tertiary education | 7 | 11.7 | 6.4 (2.9) | |
| COPD, GOLD stage | ||||
| Stage1 | 7 | 11.7 | 7.3 (4.1) | 0.003 |
| Stage 2 | 18 | 30 | 8.3 (3.8) | |
| Stage 3 | 25 | 41.7 | 10.4 (3.7) | |
| Stage 4 | 10 | 16.7 | 13.4 (3.9) | |
| MMRC Dyspnea scale | ||||
| 0 | 11 | 18.3 | 7.1 (3.5) | 0.010 |
| 1 | 16 | 26.7 | 8.8 (3.6) | |
| 2 | 23 | 38.3 | 10.2 (4.0) | |
| 3 | 7 | 11.7 | 13.6 (3.2) | |
| 4 | 3 | 5.0 | 15.7 (0.6) | |
| Night-time symptoms | ||||
| Present | 44 | 63.3 | 11.1 (3.7) | 0.432 |
| Absent | 16 | 26.6 | 8.4 (3.9) | |
PSQI: Pittsburgh Sleep Quality Index
SD: Standard deviation
One Way ANOVA and Independent-Samples T-Test for mean values of PSQI
Table 1: Baseline characteristics and mean Global PSQI score.
Sleep quality of the study population
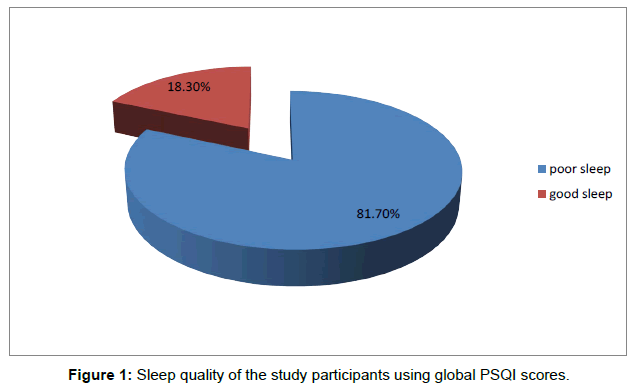
The mean global PSQI for the study subjects was 9.93 (SD 4.19, median 9.5). The mean sub scores for subjective sleep quality, sleep latency, duration, habitual sleep efficiency, sleep disturbances, sleep medications and daytime dysfunction were 1.52 (SD 0.85), 1.85 (SD 0.86), 2.07 (SD 0.82), 1.93 (SD 1.06), 1.23 (SD 0.43), 0.48 (SD 0.83) and 0.83 (SD 0.74) respectively. A significant proportion (49; 81.7%) of the study subjects rated their overall sleep quality as poor (global PSQI >5) while 11 patients (18.3%) rated their sleep quality as good as shown in Figure 1.
Description of sleep patterns according to sleep domains in the study population
Sleep quality according to sleep domains is shown in Tables 2 and 3.
| Sleep pattern | Median/ No of patients 60 | Range /Percentage % |
|---|---|---|
| Subjective sleep domain | ||
| Very good Fairly good Fairly bad Very bad |
5 28 18 9 |
8.3% 46.7% 30.0% 15.0% |
| Sleep latency domain | ||
| Latency to sleep in minutes Proportion of latency to sleep >30 min |
30.00 31 |
10.00 – 240.00 51.7% |
| Sleep duration domain | ||
| Estimated sleep time in hours | 5.00 | 2.00 - 8.00 |
| Sleep duration in hours •>7 hours •5-7 hours •<5 hours |
2 38 20 |
3.3% 63.4% 33.3% |
| Habitual sleep efficiency domain | ||
| Median sleep efficiency | 69.0% | 55.0% - 89% |
| Categories of sleep efficiency •= 85% •< 85% |
6 54 |
10.0% 90% |
PSQI: Pittsburgh Sleep Quality Index
Table 2: Sleep pattern according to sleep domains among study subjects using PSQI.
| Frequency of symptoms a week  (n (%)) N=60 |
None | <1 ce | 1 ce/2ce | = 3ce |
|---|---|---|---|---|
| Sleep disturbances | - | |||
| Waken at night Go to bathroom at night Can’t breathe well at night Cough at night Feel too hot Feel too cold Have bad dreams Have pain |
0 (0) 2 (3.3) 22 (36.7) 18 (30) 57 (95) 59 (98.3) 43 (71.7) 51 (85) |
15 (25) 11 (18.3) 15 (25) 17 (28.3) 3 (5) 0 (0) 12 (20) 0 (0) |
18 (30) 18 (30) 16 (26.7) 16 (26.7) 0 (0) 1 (1.7) 5 (8.3) 6 (10) |
27 (45.0) 29 (48.3) 7 (11.7) 9 (15.0) 0 (0) 0 (0) 0 (0) 3 (5) |
| Use of sleep medications | 42 (70) | 9 (15) | 7 (11.7) | 5 (8.3) |
| Daytime dysfunction | ||||
| Difficulty staying awake during activities In the day Lack of enthusiasm to get things done |
34 (56.7) 24 (40) |
16 (26.7) 22 (36.7) |
8 (13.3) 12 (20) |
2 (3.3) 2 (3.3) |
PSQI= Pittsburgh Sleep Quality Index
Table 3: Frequency of sleep disturbances, sleep medication use and daytime dysfunction using PSQI.
Presenting clinical symptoms
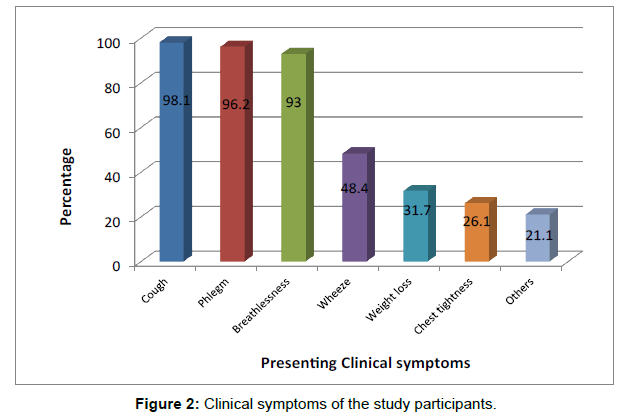
The mean duration of the first presenting respiratory symptoms in the study patients was 5.9 ± 5.7 years and ranges between 1 and 24 years (median of 4 years). Almost all the patients had cough (59, 98.1%) while less than a third had weight loss (19, 31.7%). Other symptoms included fatigue, palpitations and dyspepsia [Figure 2].
Presence of disease specific night-time symptoms in study subjects
Forty-four (73.3%) patients had disease specific symptoms at night in the past one month. Proportion of patients with disease-specific nighttime symptoms in relation to its contribution to disturbed sleep are shown in Table 4.
| Night-time symptoms | Number of patients N = 60 |
Percentage (%) |
|---|---|---|
| Most disturbing night-time symptom | ||
| Cough Breathlessness Wheeze Excess sputum Difficult to say |
22 10 4 1 7 |
36.7 16.6 6.7 1.7 11.7 |
Table 4: Presence of night-time symptoms.
Day time sleepiness in study subjects using Epworth Sleepiness Scale (ESS)
The mean score on ESS in the study patients was 5.47 ± 2.46 (Median 5.0). Three patients (5.0%) had excessive daytime sleepiness (ESS >9) while 57 patients (95%) had a normal daytime sleepiness (ESS ≤9).
Relationship between Sleep quality and severity of COPD
There were significant associations between GOLD stages of COPD and global PSQI score as well as for all sleep domains with the exception of sleep medication and habitual sleep efficiency domains [Table 5].
| Variables | GOLD stage | ||||
|---|---|---|---|---|---|
| One n= 7 | Two n=18 | Three n=25 | Four n=10 | p-value | |
| Mean Score (S.D.) | |||||
| Global PSQI | 7.3 (4.1) | 8.3 (3.8) | 10.4 (3.7) | 13.4 (3.9) | 0.003 |
| Subjective sleep quality Sleep latency Sleep duration Habitual sleep efficiency Sleep disturbances Sleep medications Day time dysfunction |
1.0 (0.8) 1.3 (0.8) 1.9 (1.1) 1.6 (1.4) 1.0 (0.0) 0.1 (0.4) 0.4 (0.5) |
1.3 (0.7) 1.7 (0.8) 1.7 (0.9) 1.6 (1.1) 1.1 (0.3) 0.3 (0.6) 0.6 (0.7) |
1.5 (0.8) 1.8 (0.9) 2.2 (0.7) 2.0 (0.8) 1.2 (0.40 0.7 (1.0) 0.9 (0.7) |
2.2 (1.0) 2.5 (0.7) 2.5 (0.5) 2.6 (0.8) 1.6 (0.5) 0.6 (0.8) 1.4 (0.7) |
0.016 0.023 0.030 0.057 0.009 0.282 0.018 |
Table 5: Global PSQI score and sleep quality domains in different COPD, GOLD stages.
Discussion
COPD negatively affects sleep and sleep impairment has been found to be a common complaint among patients with COPD contributing, to poor health status. [10] More than 80% of the patients in this study, rated their overall sleep quality as poor (PSQI >5) with a mean score of 9.93. High prevalence of impaired sleep in COPD is consistent with findings from other studies using the PSQI. Scharf et al. [11] reported 77% while Nunes et al. [12] reported a prevalence of 70%. Probably the highest prevalence of sleep impairment was in a study by Sajal De [13] in which 100% of the patients had poor sleep quality with a mean PSQI score of 11.
The median sleep latency in this study was 30 minutes with slightly more than half of patients reporting latency period beyond 30 minutes. This is longer than the normal range of 10-20 minutes indicating difficulty initiating sleep. [14] Scharf and Nimrod et al. [11,15] also found a median sleep latency of 30 minutes in their studies using PSQI. A study in which patients with COPD were compared with controls found that sleep parameters were worse in patients with COPD, including prolonged sleep latency. [16] A polysomnographic sleep study in COPD also reported prolonged sleep latency times in the excess of 30 minutes. [17]
We reported a median total sleep time of 5 hours; 96.7% of our patients had <7 hours of sleep with 33% having less than 5 hours of actual sleep. This is less than the required optimum sleep duration average of 7 hours in the adult population. [18] Habitual sleep efficiency which is the percentage of the total time in bed a person spends asleep was suboptimal. Ninety percent of the study population reported sleep efficiency of <85% with 41.7% reporting sleep efficiency of <65%. This is a significant departure from a sleep percentage of >90% which is considered normal and optimal. [18] Scharf [11] and Nimrod [15] reported a median total sleep time of 5 hours with similar sleep efficiency using PSQI. Polysomnographic sleep studies have also found that sleep efficiency and total sleep time was low among patients with COPD. [16,19] Reductions in total sleep time and sleep efficiency suggests that our patients have frequent nocturnal awakenings. This is consistent with the finding that all our patients reported nocturnal awakenings in the past one month according to the PSQI with 45% of the patients having night-time awakening ≥ 3 times per week.
A reason for this observation may be due to presence of night-time symptoms. In our study, 11.7% and 15% of patients had disturbed sleep due to breathlessness and cough at night ≥ times per week respectively. A higher percentage of patients had nocturnal symptoms in a related study in which 48% had cough and 32% had dyspnea at night ≥3 times a week. [11] However, a common observation is that cough and dyspnea were important symptoms causing sleep disturbance in both studies. We found a similar pattern of night-time symptoms to that of PSQI with cough (36.7%) being the most disturbing nocturnal symptom followed by breathlessness (16.6%) based on patients’ responses. Other studies using symptom questionnaire also reported that nocturnal symptoms especially cough and breathlessness were associated with greater likelihood of disturbed sleep. [6,20] Patients with COPD may experience nocturnal exacerbation of symptoms because lying flat during sleep impairs pulmonary mechanics and gas exchange which may lead to frequent awakening. [21]
Using the MMRC dyspnea scale, we observed that sleep worsens with increasing severity of dyspnea. Nunes et al. [22] also found that dyspnea was the best predictor of total sleep time and sleep efficiency in a study. Dyspnea is a major symptom of COPD and patients with severe dyspnea are likely to be breathless at night leading to disturbed sleep. In addition, chronic dyspnea can lead to depressive episodes leading to impaired sleep and poor quality of life although this relationship was not assessed in our study. [23] Dyspnea is also a symptom of nocturnal oxygen desaturation, which is a major determinant of frequent arousals in patients with COPD. [24]
Moreover, nocturnal oxygen desaturation may account for the nocturnal awakenings in patients without significant night-time symptoms. [25] Almost half of the respondents reported waking up to use the bathroom ≥3 times per week which could be a reflection of fragmented sleep. [26]
There was a significant association between global PSQI score; sleep domains sub scores and GOLD stage of COPD with the exception of sleep medication and habitual sleep efficiency domain. These findings suggest that COPD severity is associated with disorders of initiating and maintaining sleep as well as daytime dysfunction. Similarly, a study by Nimrod et al. [15] found a significant correlation between global PSQI score and FEV1% predicted. Some studies however found no correlation between sleep quality and lung function. [11,27] Oxygen desaturation and severity of symptoms may explain the significant association between COPD, GOLD stage and sleep quality. [1]
This study showed that 31% of our patients have used sleep medications in the last one month with 8.3% using sleep medications more than 3 days per week, an observation similar to a study by Scharf et al. [11] that reported 11.1%. The use of sleep medications by these patients shed more light on the burden of sleep disturbances in COPD and attempts made to overcome this problem.
Poor sleep quality leads to various day time dysfunctions in COPD such as daytime excessive sleepiness, neuro-cognitive decline, fatigue and impaired HRQL. [28] In the current study, 2 patients (3.3%) had lack of enthusiasm to get things done three or more times per week. This lack of enthusiasm may be due to fatigue, a common symptom in patients with COPD. [28] Fatigue imposes limitations on motivation, concentration, and the ability to engage in everyday activities such as work and household chores. [29] The PSQI may however underestimate the prevalence of fatigue because reduced motivation is just one of the aspects of fatigue. [30] Other studies have also found an association between short sleep duration and daytime fatigue. [31] Fatigue may be related to mood states such as anxiety, irritability and depression which are causes of poor sleep in COPD. [29] In a study by Kapella, [32] dyspnea, depressed mood and sleep quality had significant association with subjective fatigue.
Responses on the Pittsburgh questionnaire revealed that 2 patients (3.3%) had difficulty staying awake during activities in the day for ≥3 times per week. According to the Epworth’s sleepiness scale, only three patients (5.0%) rated daytime sleepiness as excessive (ESS >9) with a median score of 5. This suggests that despite the high prevalence of poor sleep quality, excessive daytime sleepiness does not appear to be as common in our patients. In other studies, prevalence of excessive daytime sleepiness in COPD ranged from 6.7% to 32%. Interestingly, these studies also show that the prevalence of excessive daytime sleepiness is not commensurate with the high prevalence of poor sleep quality in patients with COPD. [11,33] One of the reasons for this observation may be that COPD patients may not have excessive daytime sleepiness despite impaired sleep because of increased sympathetic nervous system activity associated with airway obstruction, hypoxia, or medications. [11]
There was a discrepancy between overall self-rated sleep quality and global PSQI score with majority of our patients (46.7%) rating their sleep quality as fairly good. In another study, 77.5% rated their sleep quality as very good or fairly good. [13] This observation suggests that patients have a poor perception of the severity of their sleep impairment. [13] Poor perception of disturbed sleep may be related to frailty and decreased acceptance of illness among patients with COPD who are mostly elderly. [34] In the index study, demographic attributes of the patients such as age, gender and BMI had minimal impact on overall sleep quality.
This study is subject to limitations. Due to the relatively small sample size, the statistical power of the study is reduced which decreases the chance of detecting significant differences when they truly exist. Recall bias is also a possible limitation, although the survey was administered by trained interviewers.
Conclusion
This study has demonstrated that patients with COPD have a high prevalence of impaired sleep quality. Presence of disease-specific night-time symptoms especially cough and dyspnea and severity of airway obstruction may contribute to impaired sleep. However, excessive daytime sleepiness is not a prominent symptom in the study population. Therefore, reducing disease- specific COPD symptoms and optimizing treatment may improve quality of sleep in COPD patients.
Ethical considerations
Approval of the Ethics and Research Committee of the Obafemi Awolowo University Teaching Hospitals Complex (OAUHC) was sought and obtained before the commencement of the study. Informed consent of the individuals for the study was obtained.
Conflict of Interest
The authors disclose that they have no conflicts of interest.
REFERENCES
- Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease. 2017 Report. GOLD Executive Summary. American Journal of Respiratory and Critical Care Medicine. 2017;195:5.
- http://www.who.int/respiratory/copd/burden/en/index.html. Accessed September 30, 201 .
- Obaseki D, Erhabor G, Burney P, Buist S, Awopeju O, Gnatiuc L. The prevalence of COPD in an African city: Results of the BOLD study, Ile-Ife, Nigeria. The European Respiratory Journal. 2013;42:932.
- Teofilo L, Lee-Chiong J. ACCP Pulmonary Medicine Board Review. (25th edition). Northbrook, Illinois (US) 60062-2348. American College of Chest Physicians. 2009. Sleep Physiology. p: 245-252.
- Colton HR, Altevogt BM. Sleep disorders and sleep deprivation: An unmet public health problem. Institute of Medicine Committee on Sleep Medicine and Research: National academies press (US): 2006. Sleep physiology Washington DC.
- Omachi TA, Blanc PD, Claman DM, Chen H, Yelin EH, Julian L, et al. Disturbed sleep among COPD patients is longitudinally associated with mortality and adverse COPD outcomes. Sleep Medicine. 2012;13:476-483.
- Agusti A, Hedner J, Marin JM, Barbe F, Cazzola M, Rennard S. Night-time symptoms: A forgotten dimension of COPD. European Respiratory Review. 2011;20:183-194.
- Fletcher CM. Standardized questionnaire on respiratory symptoms: a statement prepared and approved by the MRC Committee on Aetiology of Chronic Bronchitis (MRC Breathlessness Score) BMJ. 1960;2:1665.
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research. 1989;28:193-213.
- Stephenson JJ, Cai Q, Mocarski M, Tan H, Doshi JA, Sullivan SD. Impact and factors associated with nighttime and early morning symptoms among patients with chronic obstructive pulmonary disease. International Journal of Chronic Obstructive Pulmonary Disease. 2015;10:577-586
- Scharf SM, Maimon N, Simon-Tuval T, Bernhard-Scharf BJ, Reuveni H, Tarasiuk A. Sleep quality predicts quality of life in chronic obstructive pulmonary disease. International Journal of Chronic Obstructive Pulmonary Disease. 2010;6:1-12.
- Nunes DM, Mota RM, De Pontes Neto OL, Pereira ED, De Bruin VM, De Bruin PF. Impaired sleep reduces quality of life in chronic obstructive pulmonary disease. Lung. 2009;187:159-163.
- De S. Subjective assessment of quality of sleep in chronic obstructive pulmonary disease patient and its relationship with associated depression. Lung India. 2012;29:332-335.
- Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. A meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255-1273.
- Nimrod M, Tsachit ST, Ariel T, Haim R, Barbara BS, Steven MS. Sleep Disturbances and Health Related Quality of Life (HrQOL) In Patients With COPD. D45 Chronic Obstructive Pulmonary Disease Comorbidity: American Thoracic Society. p. A5931.
- Azuma M, Chin K, Yoshimura C, Takegami M, Takahashi K, Sumi K, et al. Associations among chronic obstructive pulmonary disease and sleep-disordered breathing in an urban male working population in Japan. Respiration 2014;88:234-243.
- McSharry DG, Ryan S, Calverley P, Edwards JC, McNicholas WT. Sleep quality in chronic obstructive pulmonary disease. Respirology. 2012;17:1119-1124.
- Spriggs WH. Normal sleep: Essentials of Polysomnography. 2nd edition. Burlington, MA 01803. Jones and Bartlett learning. 2014;1-11.
- Lofdahl M, Theorell-Haglow J, Olafsdottir IS, Lindberg E, Jansson C. Recorded quality of sleep in patients with copd. Chest. 2008;134 p20002.
- Miravitlles M, Worth H, Soler Cataluña J, Price D, De Benedetto F, Roche N, et al. Observational study to characterise 24-hour COPD symptoms and their relationship with patient-reported outcomes: results from the ASSESS study. Respir Res. 2014;15:1-13.
- Mohsenin V. Sleep in chronic obstructive pulmonary disease. Seminars in Respiratory and Critical Care Medicine. 2005;26:109-116.
- Nunes DM, De Bruin VM, Louzada FM, Peixoto CA, Cavalcante AG, Castro-Silva C, et al. Actigraphic assessment of sleep in chronic obstructive pulmonary disease. Sleep Breath. 2013;17:125-132.
- MartÃÂnez Francésa ME, Perpiñá Torderaa M, Belloch Fusterb A, MartÃÂnez Moragónc EM, Compte Torreroa L. Impact of baseline and induced dyspnea on the quality of life of patients with COPD. Archivos de BronconeumologÃÂa (English Version). 2008;44:127-134.
- Zanchet RC, Viegas CA. Nocturnal desaturation: predictors and the effect on sleep patterns in patients with chronic obstructive pulmonary disease and concomitant mild daytime hypoxemia. J Bras Pneumol. 2006;32:207-212.
- Doghramj K. Insomnia and comorbid chronic obstructive pulmonary disease. Medscape education. From Medscape Neurology. 2008.
- Bliwise DL, Foley DJ, Vitiello MV, Ansari FP, Ancoli-Israel S, Walsh JK. Nocturia and disturbed sleep in the elderly. Sleep Medicine. 2009;10:540-548.
- Hynninen MJ, Pallesen S, Nordhus IH. Factors affecting health status in COPD patients with co-morbid anxiety or depression. International Journal of Chronic Obstructive Pulmonary Disease. 2007;2:323-328.
- http://www.intechopen.com/books/copd-clinical-perspectives/chronic-obstructive-pulmonary-disease-and-sleep-quality. Assessed November 11, 2014.
- Wong CJ, Goodridge D, Marciniuk DD, Rennie D. Fatigue in patients with COPD participating in a pulmonary rehabilitation program. International Journal of Chronic Obstructive Pulmonary Disease. 2010;5:319-326.
- Smets EM, Garssen B, Bonke B, De Haes JC. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. Journal of Psychosomatic Research. 1995;39:315-325.
- Goldman SE, Ancoli-Israel S, Boudreau R, Cauley JA, Hall M, Stone KL, et al. Sleep problems and associated daytime fatigue in community-dwelling older individuals. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2008;63:1069-1075.
- Kapella MC, Larson JL, Patel MK, Covey MK, Berry JK. Subjective fatigue, influencing variables, and consequences in chronic obstructive pulmonary disease. Nursing Research. 2006;55:10-17.
- Ali Zohal M, Yazdi Z, Kazemifar AM. Daytime sleepiness and quality of sleep in patients with COPD compared to control group. Global Journal of Health Science. 2013;5:150-155.
- Uchmanowicz I, Jankowska-Polanska B, Chabowski M, Uchmanowicz B, Fal AM. The influence of frailty syndrome on acceptance of illness in elderly patients with chronic obstructive pulmonary disease. International Journal of COPD. 2016;11:2401-2407.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.