Prospect of Brain‑Machine Interface in Motor Disabilities: The Future Support for Multiple Sclerosis Patient to Improve Quality of Life
- *Corresponding Author:
- Dr. Ajay Vikram Singh
Center for Biotechnology and Interdisciplinary Studies, Room 2127, Rensselaer Polytechnic Institute, 110 8th Street, Troy, NY 12180, USA.
E-mail: singha8@rpi.edu
Citation: Khare M, Singh AV, Zamboni P. Prospect of brain-machine interface in motor disabilities: The future support for multiple sclerosis patient to improve quality of life. Ann Med Health Sci Res 2014;4:305-12.
Abstract
Multiple sclerosis (MS) is an autoimmune neurological disorder, which has impacted health related quality of life (HRQoL) more intensively than any other neurological disorder. The approaches to improve the health standard in MS patient are still a subject of primary importance in medical practice and seek a lot of experimental exploration. The present review briefly explains the anomaly in neuron anatomy and dysfunction in signal transmission arising in the context with the chronic cerebrospinal venous insufficiency (CCSVI), a recent hypothesis related with MS pathophysiology. Subsequently, it insights brain‑machine interface (BMI) as an alternative approach to improve the HRQoL of MS subjects. Information sources were searched from peer‑reviewed data bases (Medline, BioMed Central, PubMed) and grey‑literature databases for data published in 2000 or later. We also did systemic search in edited books, articles in seminar papers, reports extracted from newspapers and scientific magazines, articles accessed from internet; mostly using PubMed, Google search engine and Wikipedia. Out of approximately 178, 240 research articles obtained using selected keywords, those articles were included in the present study which addresses the latest definitions of HRQol and latest scientific and ethical developments in the research of MS and BMI. The article presented a brief survey of CCSVI mediated MS and BMI‑approach as a treatment to serve the patients suffering from disabilities as a result of MS, followed by successful precedence of BMI approach. Apart from these, the major findings of selected research articles including the development of parameters to define HRQoL, types and development of BMIs and its role in interconnecting brain with actuators, along with CCSVI being a possible cause of MS have formed the foundations to conclude the findings of the present review article. We propose a perspective BMI approach and promises it holds for future research to improve HRQoL in MS patients. In addition, we propose that brain‑computer interfaces will be the core of new treatment modalities in the future for MS disabilities.
Keywords
Blood brain barrier, Brain-machine interface, Chronic cerebrospinal venous insufficiency, Immunomodulation, Multiple sclerosis
Introduction
Multiple sclerosis (MS) is an autoimmune disease of the nervous system which affects the brain and the spinal cord.[1] MS is caused primarily due to the protective covering of myelin sheath surrounding the nerve cells getting scarred (hence sclerosis) or damaged; causing the nerve signals to slow down or stop.[1] Depending on the otherwise destination of the scarred nerve cell, this may lead to single or multiple deconditions including motor disabilities, speech disabilities, swallowing disabilities, visual disabilities, bladder constipation and other neuronal problems.[2,3] These deconditions hence take a toll at the patient’s physiological functions, leaving him practically dependent on family, friends and well-wishers for care and for even most basic parameters of a healthy life; viz. level of fitness, self-image, satisfaction with family-life, work, interaction with other people, social-support and life in general.[4-12] As these parameters being subjective in nature; a more objective parameter termed as quality of life (QoL) in general or, health related quality of life (HRQoL) in specific, is coined up to analyze and study the health related effects of any medical approach toward a patient. A patient being better off or worse off in terms of HRQoL by a particular approach defines the success or failure of alternative theranostic approaches available in medical practice and the continuous development of diagnostics and therapeutic tools.[13] This is one fact justifying the concern over QOL of MS patients.[13] Furthermore, HRQoL has been more intensively studied in MS than in any other neurological disorder.[12]
In present article’s framework, focus is laid on the factors suggesting the possible improvement in QoL (or HRQoL) if contribution of brain-machine interface (BMI) can be tapped to the treatment of patients suffering from motor disabilities and thus facing problems in moving their arms or legs, in walking and problems with coordination and making small movements. The aspiration with BMI finds its roots in the precedence that exemplifies their successful exploration in the past.[14-18]
Materials and Methods
To keep updated with and to include the latest definitions of HRQoL; research developments in QoL related studies in case of MS, since January 2000 have been included for the present study. As an exception to this, such primary research articles have also been selected which have laid the foundation of BMI and those which have brought the pathogenesis of MS into light of chronic cerebrospinal venous insufficiency (CCSVI). On the basis of these selection criteria, 56 research articles out of 178, 240 articles obtained from PubMed, BioMed Central, MedLine and Google-search engine using keywords “HRQoL,” “MS,” “Neurodegenerative diseases,” “BMI,” “BMI in motor disabilities” and “motor prosthesis,” have been selected. Apart from these articles, reference books and 12 website resources have also been included in the present study.
Result of the search
The results of the search are summarized as presented in the following Table 1:
| Author (year) | Sample size | Study design | Challenge assessed |
|---|---|---|---|
| Romberg (2005) | 95 | Research on primary data; experimental study | Contribution of long-term exercise in improvingfunctional impairment and HRQoL |
| Mitchell (2005) | - | Review and amalgamation of existing definitionsand parameters for measuring HRQoL | Parameters to measure HRQoL in subjects with MS |
| Nicolelis (2006) | - | - | Types and development of brain-machine interface |
| Nicolelis (2009) | - | Experimental in-vivo study | Direct connection between brain and actuators |
| Zamboni andSingh (2009) | - | - | CCSVI as a possible cause of MS |
| Haggard (2006) | ~56 | Experimental in vivo study | Possibility of brain being used as fingers of the hand |
Table 1: Result of seminal literature-survey
Anatomy of neuron/axon
Most of the neurons consist of the cell body (or soma), the nerve fiber (or axon) and the receiving processes (or dendrites).[19] Among these, axon is a cable like projection arising from the soma and carrying away the nerve impulses from soma to dendrites to conduct toward other neurons. Large axons acquire an insulating sheath of myelin and are known as myelinated fiber.[19] This myelin sheath insulates and protects axons in the vertebrate nervous system. Myelinated axons conduct neural responses, also known as action potential, much more rapidly and efficiently than unmyelinated axons. Myelin is made by specialized glial cells which form myelin by wrapping their membranes around axons many times and hence that the myelin matures to form a compact sheath.
Transmission of neuronal signal
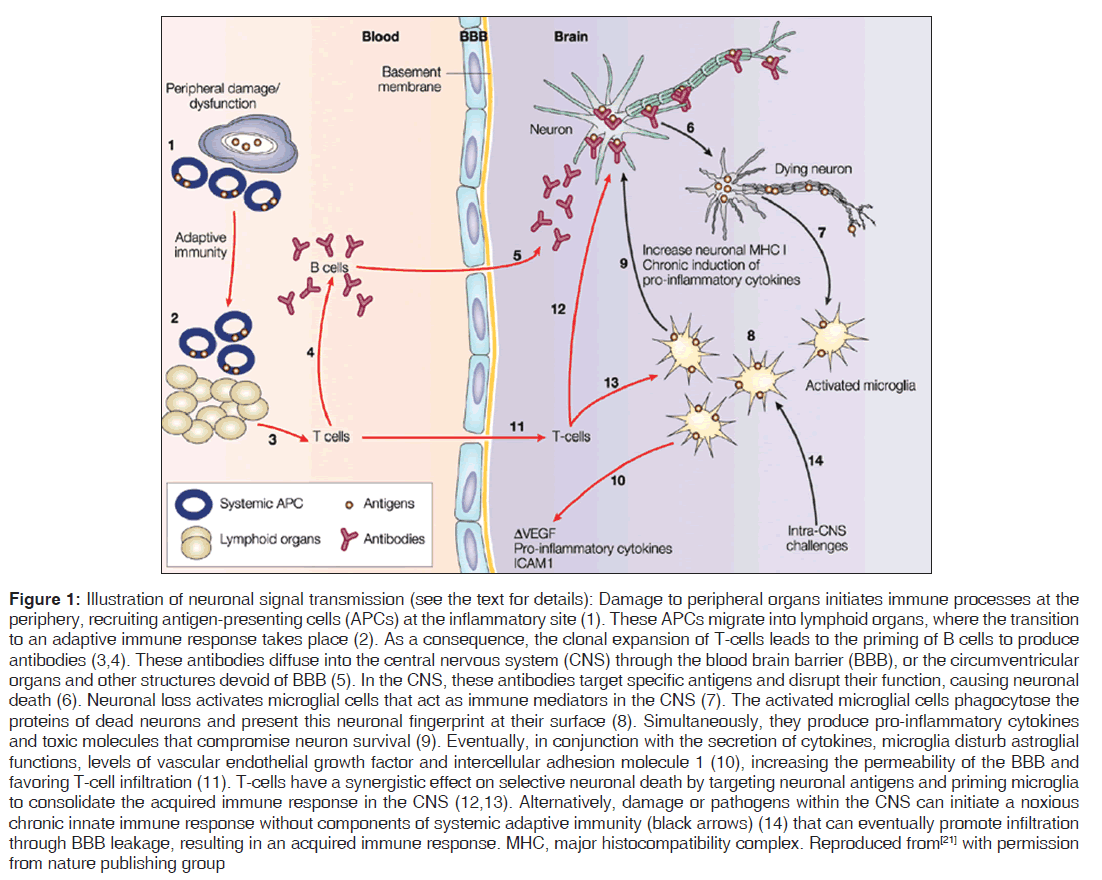
The myelin coating consists of small unmyelinated sections of the membrane, called nodes of Ranvier. The voltage-gated sodium ions responsible for carrying the action potential are clustered at these nodes and excluded from the myelinated internodes.[19,20] The organization of myelinated axons into the different domains – short nodes with sodium channels and long myelin internodes with low membrane capacitance – allows rapid and efficient conduction of action potentials [Figure 1].[19-21]
Figure 1: Illustration of neuronal signal transmission (see the text for details): Damage to peripheral organs initiates immune processes at the periphery, recruiting antigen-presenting cells (APCs) at the inflammatory site (1). These APCs migrate into lymphoid organs, where the transition to an adaptive immune response takes place (2). As a consequence, the clonal expansion of T-cells leads to the priming of B cells to produce antibodies (3,4). These antibodies diffuse into the central nervous system (CNS) through the blood brain barrier (BBB), or the circumventricular organs and other structures devoid of BBB (5). In the CNS, these antibodies target specific antigens and disrupt their function, causing neuronal death (6). Neuronal loss activates microglial cells that act as immune mediators in the CNS (7). The activated microglial cells phagocytose the proteins of dead neurons and present this neuronal fingerprint at their surface (8). Simultaneously, they produce pro-inflammatory cytokines and toxic molecules that compromise neuron survival (9). Eventually, in conjunction with the secretion of cytokines, microglia disturb astroglial functions, levels of vascular endothelial growth factor and intercellular adhesion molecule 1 (10), increasing the permeability of the BBB and favoring T-cell infiltration (11). T-cells have a synergistic effect on selective neuronal death by targeting neuronal antigens and priming microglia to consolidate the acquired immune response in the CNS (12,13). Alternatively, damage or pathogens within the CNS can initiate a noxious chronic innate immune response without components of systemic adaptive immunity (black arrows) (14) that can eventually promote infiltration through BBB leakage, resulting in an acquired immune response. MHC, major histocompatibility complex. Reproduced from[21] with permission from nature publishing group
Immunomodulation and cerebrospinal venous insufficiency: New actors in MS pathophysiology
Main and the most commonly identified cause of MS is the disruption of myelin sheath; mainly due to attack by autoimmune system of the vertebrate. In diseases of myelin rupture, neurological dysfunction results both from impeded axonal conduction and from axonal damage that results when glial cells are lost. Lately, iron overload and oxidative stress have been demonstrated as surrounding cause, which leads to immunomodulation in chronic conditions in MS.[22] Iron overload has been demonstrated in MS lesions, as a feature common with other neurodegenerative disorders. However, the recent description of CCSVI associated to MS, with significant anomalies in cerebral venous outflow hemodynamics, permit to propose a parallel with chronic venous disorders in the mechanism of iron deposition.[23,24] Abnormal cerebral venous reflux is peculiar to MS and was not found in a miscellaneous of patients affected by other neurodegenerative disorders characterized by iron stores, such as Parkinson’s, Alzheimer’s, amyotrophic lateral sclerosis. Several recently published cellular, radiological and histopathological studies support the hypothesis that MS progresses along the venous vasculature and oxidative stress plays a central role in this perspective.[25-28] The peculiarity of CCSVI-related cerebral venous blood flow disturbances, together with the histology of the perivenous spaces and recent findings from advanced magnetic resonance imaging techniques, support the hypothesis that iron deposits in MS are a consequence of altered cerebral venous return and chronic insufficient venous drainage.[29] The hypothesis is further corroborated by recent findings demonstrating genetic pre-disposition through polymorphisms in the genes coding for iron binding and transporting proteins in MS etiopathogenesis and custom CGH array profiling of copy number variations on chromosome 6p21.32 (HLA locus) in patients with venous malformations.[30,31] An open-label study of endovascular treatment of MS patients with CCSVI through percutaneous transluminal angioplasty which is termed as “liberation therapy” has shown positively influenced clinical and QOL parameters of the associated MS compared with the preoperative assessment.[32]
Perspective of BMI/brain-computer interface (BCI) to improve HRQoL in MS
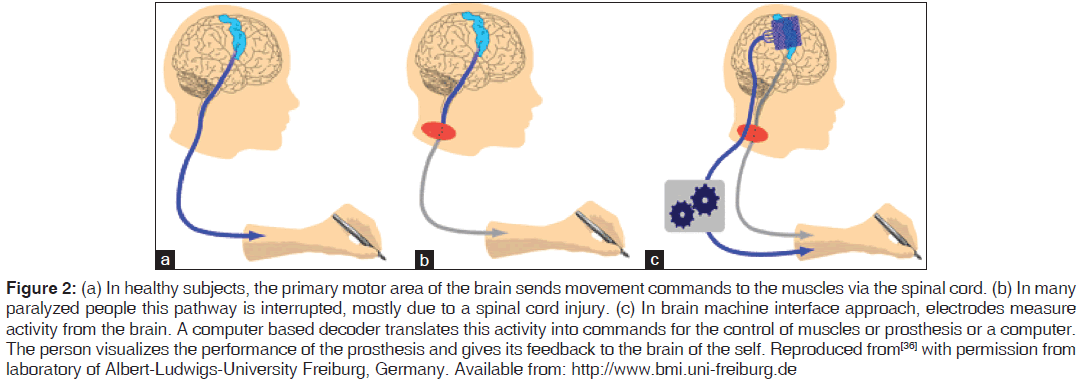
A BCI or a BMI, is a direct communication pathway between the brain and an external device. BMIs are often directed at assisting, augmenting, or repairing human cognitive or sensory-motor functions.[33-35] The brain signals are picked up from inside the skull using invasive methods or from the surface of the skull using non-invasive methods and passed on to a machine or prosthetic device in case of paralyzed patients or person with amputed arms or legs [Figure 2].[33,34] These prosthetic devices decode these fed electrical signals and perform the corresponding motor function. This interface helps in understanding how the brain controls movement and thus gives rise to better medical technology for paralyzed people.[36,37]
Figure 2: (a) In healthy subjects, the primary motor area of the brain sends movement commands to the muscles via the spinal cord. (b) In many paralyzed people this pathway is interrupted, mostly due to a spinal cord injury. (c) In brain machine interface approach, electrodes measure activity from the brain. A computer based decoder translates this activity into commands for the control of muscles or prosthesis or a computer. The person visualizes the performance of the prosthesis and gives its feedback to the brain of the self. Reproduced from[36] with permission from laboratory of Albert-Ludwigs-University Freiburg, Germany. Available from: http://www.bmi.uni-freiburg.de
In last decade, research and advances made in BMI/BCI provide a direct interface between a brain and a machine/ computer. BMI involves monitoring real-time brain activity, detecting characteristic voluntary brain pattern or brain-waves’ alterations in real-time (that the user utilizes to communicate with the outside world), an algorithm to translate these patterns of brain into a coded control to be given to a machine, feedback of the performance of the machine due to this input control signal into the brain (most commonly, by seeing the performance of the machine). This feedback to the brain helps the brain to further improve next array of brain pattern for the task on board. So strictly, a BMI must fulfill the following four criteria to function as a real-time interface between machine and brain:[38,39]
1. Direct: The system must rely on activity recorded directly from the brain
2. Intentional control: At least one recordable brain signal, which can be intentionally modulated, must provide input to the BCI (electrical potentials, magnetic fields or hemodynamic changes)
3. Real time processing: The signal processing must occur online and yield a communication or control signal
4. Feedback: The user must obtain feedback about the success or failure of his/her efforts to communicate or control.
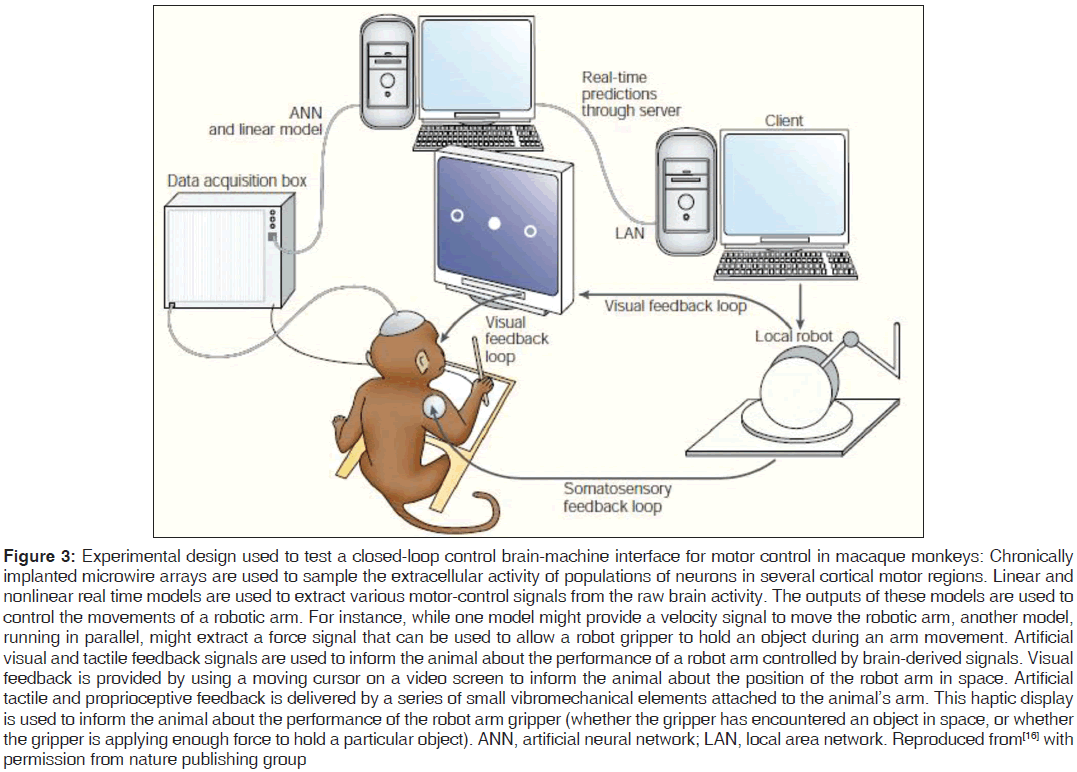
For practical realization of BMI approach to be successful in improving the QoL of persons suffering from motor-disabilities, in general, the approach is required to accommodate, distinguish, interpret and obey the multiple signals and their respective control for multi-movements simultaneously [Figure 3]. For example, a person should be able to not only grip and clutch an object but also to move it in the desired direction. Simultaneous commands for these simultaneous movements to be fed in the machine from the brain as an interface at input end can be tapped from specific locations (primary motor cortex, supplementary motor cortex, premotor cortex, posterior parietal cortex, secondary motor and sensory area) of the brain, which are responsible for motor-signals resulting in voluntary movements in otherwise healthy person.[40] This approach results in helping alternatively a disabled person in grasping a pen and writing with it simultaneously.[41-43] Successful results of multi-movements using a BMI/BCI have been reported for experiments on macaque monkeys where activities of the motor region of the animal’s brain are extracted in real-time, analyzed and interpreted by artificial neural network model, fed as input to a robotic arm to grip an object and move it in space and performance of the robotic arm was visually feedback in the brain of the monkey.[16]
Figure 3: Experimental design used to test a closed-loop control brain-machine interface for motor control in macaque monkeys: Chronically implanted microwire arrays are used to sample the extracellular activity of populations of neurons in several cortical motor regions. Linear and nonlinear real time models are used to extract various motor-control signals from the raw brain activity. The outputs of these models are used to control the movements of a robotic arm. For instance, while one model might provide a velocity signal to move the robotic arm, another model, running in parallel, might extract a force signal that can be used to allow a robot gripper to hold an object during an arm movement. Artificial visual and tactile feedback signals are used to inform the animal about the performance of a robot arm controlled by brain-derived signals. Visual feedback is provided by using a moving cursor on a video screen to inform the animal about the position of the robot arm in space. Artificial tactile and proprioceptive feedback is delivered by a series of small vibromechanical elements attached to the animal’s arm. This haptic display is used to inform the animal about the performance of the robot arm gripper (whether the gripper has encountered an object in space, or whether the gripper is applying enough force to hold a particular object). ANN, artificial neural network; LAN, local area network. Reproduced from[16] with permission from nature publishing group
Implication of BMI in MS patients
In patients with MS and other diseases of myelin, therapies which promote myelin repair might lead to clinical benefit by restoring fast axonal conduction and preventing irreversible damage to axons.[44-47] However, there have been reports of failure of remyelination in different models of MS.[48-50] As an alternative approach for the treatment of patients suffering from MS and resulting in inability to use their arms and legs due to no movement or movement being relatively constrained; prospects of BMI approach as mentioned above are explored here.
The added incentive for looking into BMI in the case of an MS patient lies in the fact that unlike an amputated organ say arm or leg; the mere availability of organ, although nonresponsive to otherwise voluntarily exercised control of the patient, soothes the communication between the person’s brain and corresponding organ. This communication always remains the foremost hurdle in BMI,[51] which is not at all an issue in case of MS patients. It has already been possible for paralyzed patients to communicate and move around using computers that sense brain waves.[52,53]
Attempts have also been made to computer-enhance the nervous system of a healthy human and to make a computer detect patterns of electrical signals, which correspond to movement and to send those signals to actuators.[54-56] These models mimic the real-life behavior of neurons and the electrical messages they produce between input (such as nerve endings in the hand), brain- processing and the output from the brain (such as responding to nerve signals as an actuator giving motor-functions).[57]
In case of motor-disabled MS patient incapable to use his arms/ legs for daily chores and hence being totally or partly dependent on others, the actuator to be used is the patient’s own actual organ. Thus to implement this in practice, the appropriate approach lies in possibility of reverse-engineering the modus-operandi of the experiments based on nerve-computer circuit that joins single neurons and computer chips.[58,59] In the experimental proof of concept, the circuit was able to send a discrete signal from a computer chip to a neuron, from that neuron to second neuron in a network and from the second neuron back to the computer chip.[58,59]
The crux of BMI implication in MS lies in inability of neurons to transmit the electrical impulses through its scarred axons. To a degree, the MS patient’s computer-nerve circuit may have computer chips enabled to detect patterns of electrical signals responsible for movement,[54,58] from the location of axon-hillock.[19] This electrical impulse-signal instead of getting transmitted through neurons may be transmitted through these computer chips up to the “actuator,” say hands here; as transmitted by some human brain machine interface devices.[60] As the patient’s hands were able to receive, decode and interpret the neuronal signals corresponding to movement, gripping and other motor activities and hence was able to use his hands well before damage to neurons. Thus, the hands are expected to not find any problem in communicating with neuronal signals being transmitted through computer chips, as circuit signals.
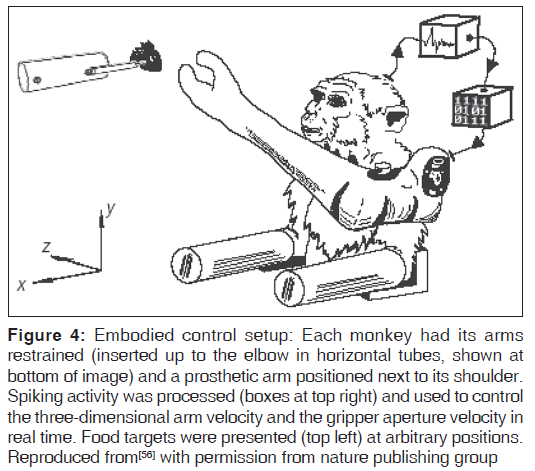
The BMI approach has been successful to varying extents in the past in several cases.[15-19,52-56] Monkeys have been successfully trained to use robotic arms [Figure 4] and people have been able to successfully use simple prototypes to write short texts or control technical devices.[55,56,61]
Figure 4: Embodied control setup: Each monkey had its arms restrained (inserted up to the elbow in horizontal tubes, shown at bottom of image) and a prosthetic arm positioned next to its shoulder. Spiking activity was processed (boxes at top right) and used to control the three-dimensional arm velocity and the gripper aperture velocity in real time. Food targets were presented (top left) at arbitrary positions. Reproduced from[56] with permission from nature publishing group
An exciting achievement of BMI is reflected in the functioning of “brainfingers,”[62-64] where a motor-disabled patient wears a headband which detects electrical signals from the facial muscles, eye movements and brainwaves. The software decodes these signals into virtual fingers or Brainfingers, which trigger mouse and keyboard events to control third party software such as Ezkeys, Reach, IntelliTools, Dynavox Windows software, The grid, gaze talk, etc., The brain fingers solution can include head-tracking devices such as SmartNav.[62]
In very near future, the Brazilian world cup 2014 is expected to witness one of the at-one-time-unimaginable successes of BMI turning into reality. One of the highlights of the ceremony will see the Brazilian team walk on to the pitch, striding out behind two quadriplegic teenagers. These quadriplegic teenagers, wearing exoskeletons controlled by BMI technology are entrusted to then approach and kick the ball.[65]
Conclusion
The precedence of success of BMI mentioned above colors the hope of this approach to be the next therapy for MS-disabled patients to depend upon to a greater extent. To be able to live up to these expectations, some areas of BMI need to be explored further. The Machine should be able to distinguish between the signals of concern and noise-signals/evil-signals for any specific task. Hence the interface should not direct the car to run in a zig-zag manner, even if the user’s brain momentarily kids of doing so. This requires a shared – control between the user and the interfaced Machine.
As the signal transferred through BMI passes a series of algorithms and gets better after feedback, the rate of transfer of intention into action is sometimes too slow than required. Therefore, BMI performance would be particular challenging to accomplish reflexes against involuntary and nearly instantaneous movement in response to a sudden environmental stimulus. Like so to rely on BMI that it will work if accidentally some acid falls on the hand and one wants to pull his hand back at once is out of question, unless a signal can be made as quickly responsive as a central nervous system’ neuron (which makes us pull back our hand if touched a hot plate accidentally). In order to obtain the near natural response from practical BMIs, the otherwise hindrance would have been the stepper-motor-type performance of prosthetic device or robotic arm or leg; which is not at all required in case of MS patient as the organ itself receives the neuronal signal through the BMI. Once it gets adapted to the relation between his intentions and the device’s translation to accomplish these intentions. This claims the higher success of BMI possible in case of MS patients when compared to patients disabled due to amputation or otherwise.
This approach will enable MS patients to conduct routine activities of varying complexities by themselves. At the same time, as it will increase the HRQoL of MS patients, it promises new tools to combat the inadequacies of present medical support and hence will enrich the medical therapy as a whole by exposing the better tools to help MS patients.
Acknowledgements
AVS gratefully acknowledges funding support from Department of Biomedical Engineering (BME), Rensselaer Polytechnic Institute (RPI).
References
- Calabresi P. Multiple sclerosis and demyelinating conditions of the central nervous system. In: Goldman L, Ausiello D, editors. Cecil Medicine. 23rd ed., Ch. 436. Philadelphia, Pa: Saunders Elsevier; 2007.
- National Library of Medicine [http://www.nlm.nih. gov/medlineplus/encyclopedia.html]. Maryland: National Institute of Health; c1997-2012. Available from http://www.vsearch.nlm.nih.gov/vivisimo/ cgi-bin/query-meta?v%3Aproject=medlineplus and query=multiple+sclerosis. [Last cited on 2013 Oct 04].
- Wikipedia Free Encyclopaedia [www.wikipedia.org]. The encyclopedia. Available from: http://www.en.wikipedia. org/wiki/Multiple_sclerosis. [Last updated on 2013 Sep 28; Last cited on 2013 Oct 04].
- Opara JA, Jacarz K, Brola W. Quality of life in multiple sclerosis. J Neurol Sci 2002;205:51-8.
- Opara J, Jaracz K, Brola W. Current possibilities of assessment of quality of life in multiple sclerosis. Neurol Neurochir Pol 2006;40:336-41.
- Romberg A, Virtanen A, Ruutiainen J. Long-term exercise improves functional impairment but not quality of life in multiple sclerosis. J Neurol 2005;252:839-45.
- Fischer JS, Priore RL, Jacobs LD, Cookfair DL, Rudick RA, Herndon RM, et al. Neuropsychological effects of interferon beta-1a in relapsing multiple sclerosis. Multiple Sclerosis Collaborative Research Group. Ann Neurol 2000;48:885-92.
- Freeman JA, Thompson AJ, Fitzpatrick R, Hutchinson M, Miltenburger C, Beckmann K, et al. Interferon-beta1b in the treatment of secondary progressive MS: Impact on quality of life. Neurology 2001;57:1870-5.
- Henriksson F, Fredrikson S, Masterman T, Jönsson B. Costs, quality of life and disease severity in multiple sclerosis: A cross-sectional study in Sweden. Eur J Neurol 2001;8:27-35.
- Langdon DW, Thompson AJ. Multiple sclerosis: A preliminary study of selected variables affecting rehabilitation outcome. Mult Scler 1999;5:94-100.
- Vermersch P, de Seze J, Delisse B, Lemaire S, Stojkovic T.Quality of life in multiple sclerosis: Influence of interferon-beta1 a (Avonex) treatment. Mult Scler 2002;8:377-81.
- Mitchell AJ, Benito-León J, González JM, Rivera-Navarro J. Quality of life and its assessment in multiple sclerosis: Integrating physical and psychological components of wellbeing. Lancet Neurol 2005;4:556-66.
- Institut du Cerveau et de la Moelle epiniere [www. icm-institute.org]. France: Brain and Spine Institute; c1997-2012. Available from: http://www.icm-institute. org. [Last updated on 2013 Sep 17; Last cited on 2013 Oct 04].
- Lebedev MA, Nicolelis MA. Brain-machine interfaces: Past, present and future. Trends Neurosci 2006;29:536-46.
- Nicolelis MA. Actions from thoughts. Nature 2001;409:403-7.
- Nicolelis MA. Brain-machine interfaces to restore motor function and probe neural circuits. Nat Rev Neurosci 2003;4:417-22.
- Chapin JK. Neural prosthetic devices for quadriplegia. Curr Opin Neurol 2000;13:671-5.
- Mussa-Ivaldi FA, Miller LE. Brain-machine interfaces: Computational demands and clinical needs meet basic neuroscience. Trends Neurosci 2003;26:329-34.
- Fine C, editor. The Brittanica Guide to the Brain. London: Running Press Book Publishers; 2008. Ch. How the Brain Works-( Anatomy of a Neuron, How Information is Transmitted). p 27-41.
- The Talbot Lab [www.talbotlab.stanford.edu]. Stanford, CA: Stanford University School of Medicine. Available from: http://www.talbotlab.stanford.edu/projects. html#nodes. [Last updated on 2013 Aug 10; Last cited on 2013 Oct 04].
- Nguyen MD, Julien JP, Rivest S. Innate immunity: The missing link in neuroprotection and neurodegeneration? Nat Rev Neurosci 2002;3:216-27.
- Zamboni P. The big idea: Iron-dependent inflammation in venous disease and proposed parallels in multiple sclerosis. J R Soc Med 2006;99:589-93.
- Singh AV. Multiple sclerosis takes venous route: CCSVI and liberation therapy. Indian J Med Sci 2010;64:337-40.
- Singh AV, Zamboni P. Anomalous venous blood flow and iron deposition in multiple sclerosis. J Cereb Blood Flow Metab 2009;29:1867-78.
- Tisato V, Zauli G, Voltan R, Gianesini S, di Iasio MG, Volpi I, et al. Endothelial cells obtained from patients affectedby chronic venous disease exhibit a pro-inflammatory phenotype. PLoS One 2012;7:e39543.
- Coen M, Menegatti E, Salvi F, Mascoli F, Zamboni P, Gabbiani G, et al. Altered collagen expression in jugular veins in multiple sclerosis. Cardiovasc Pathol 2013;22:33-8.
- Zamboni P, Menegatti E, Conforti P, Shepherd S, Tessari M, Beggs C. Assessment of cerebral venous return by a novel plethysmography method. J Vasc Surg 2012;56E1:677-85.
- Uttara B, Singh AV, Zamboni P, Mahajan RT. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol 2009;7:65-74.
- Zamboni P, Menegatti E, Galeotti R, Malagoni AM, Tacconi G, Dall’Ara S, et al. The value of cerebral Doppler venous haemodynamics in the assessment of multiple sclerosis. J Neurol Sci 2009;282:21-7.
- Gemmati D, Zeri G, Orioli E, De Gaetano FE, Salvi F, Bartolomei I, et al. Polymorphisms in the genes coding for iron binding and transporting proteins are associated with disability, severity, and early progression in multiple sclerosis. BMC Med Genet 2012;13:70.
- Ferlini A, Bovolenta M, Neri M, Gualandi F, Balboni A, Yuryev A, et al. Custom CGH array profiling of copy number variations (CNVs) on chromosome 6p21.32 (HLA locus) in patients with venous malformations associated with multiple sclerosis. BMC Med Genet 2010;11:64.
- Zamboni P, Galeotti R, Menegatti E, Malagoni AM, Gianesini S, Bartolomei I, et al. A prospective open-label study of endovascular treatment of chronic cerebrospinal venous insufficiency. J Vasc Surg 2009;50:1348-581.
- Wikipedia Free Encyclopaedia [www.wikipedia.org]. The encyclopedia. Available from: http://www.en.wikipedia. org/wiki/Brain_computer_interface. [Last updated on 2013 Sep 30; Last cited on 2013 Oct 04].
- Vidal J. Real-time detection of brain events in EEG. Proceedings of The IEEE 1977;65:633-41. doi:10.1109/ PROC.1977.10542.
- Vidal JJ. Toward direct brain-computer communication. Annu Rev Biophys Bioeng 1973;2:157-80.
- Brain machine interfacing initiative [www.bmi.uni-freiburg. de/]. Freiburg: BMII; c2006-2013. Available from: http:// www.bmi.uni-freiburg.de/. [Last cited on 2013 Oct 04].
- Adaptive Control of Motor Prosthesis [www.metacomp.huji. ac.il/]. Jerusalem: METACOMP. Available from: http:// www.metacomp.huji.ac.il/. [Last updated on 2012 Nov 12; Last cited on 2013 Oct 04].
- Wolpaw JR, Birbaumer N, McFarland DJ, Pfurtscheller G, Vaughan TM. Brain-computer interfaces for communication and control. Clin Neurophysiol 2002;113:767-91.
- Pfurtscheller G, Allison BZ, Brunner C, Bauernfeind G, Solis-Escalante T, Scherer R, et al. The hybrid BCI. Front Neurosci 2010;4:30.
- Fine C, editor. The Brittanica Guide to the Brain. London: Running Press Book Publishers; 2008. Ch. How the Brain Works-(Movement). P 52-8.
- Pfurtscheller G, Müller-Putz GR, Schere R, Neuper C. Rehabilitation with brain-computer interface systems. IEEE Comput Sci 2008;41:58-65.
- Pfurtscheller G, Guger C, Müller G, Krausz G, Neuper C. Brain oscillations control hand orthosis in a tetraplegic. Neurosci Lett 2000;292:211-4.
- Obermaier B, Müller GR, Pfurtscheller G. “Virtual keyboard” controlled by spontaneous EEG activity. IEEE Trans Neural Syst Rehabil Eng 2003;11:422-6.
- Smith KJ, Blakemore WF, McDonald WI. Central remyelination restores secure conduction. Nature 1979;280:395-6.
- Jeffery ND, Blakemore WF. Locomotor deficits induced by experimental spinal cord demyelination are abolished by spontaneous remyelination. Brain 1997;120 (Pt 1):27-37.
- Yao DL, Liu X, Hudson LD, Webster HD. Insulin-like growth factor I treatment reduces demyelination and up-regulates gene expression of myelin-related proteins in experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A 1995;92:6190-4.
- Cannella B, Hoban CJ, Gao YL, Garcia-Arenas R, Lawson D, Marchionni M, et al. The neuregulin, glial growth factor 2, diminishes autoimmune demyelination and enhances remyelination in a chronic relapsing model for multiple sclerosis. Proc Natl Acad Sci U S A 1998;95:10100-5.
- Stangel M, Compston A, Scolding NJ. Polyclonal immunoglobulins for intravenous use do not influence the behaviour of cultured oligodendrocytes. J Neuroimmunol 1999;96:228-33.
- Targett MP, Sussman J, ScoldingN, O’Leary MT, Compston DA, Blakemore WF. Failure to achieve remyelination of demyelinated rat axons following transplantation of glial cells obtained from the adult human brain. Neuropathol Appl Neurobiol 1996;22:199-206.
- Keirstead HS, Blakemore WF. Identification of post-mitotic oligodendrocytes incapable of remyelination within the demyelinated adult spinal cord. J Neuropathol Exp Neurol 1997;56:1191-201.
- Craelius W. The bionic man: Restoring mobility. Science 2002;295:1018-21.
- Barinaga M. Turning thoughts into actions. Science 1999;286:888-90.
- Wickelgren I. Neuroscience. Tapping the mind. Science 2003;299:496-9.
- Wikipedia Free Encyclopaedia [www.wikipedia.org]. The encyclopedia. Available from: http://www.en.wikipedia. org/wiki/Kevin_Warwick. [Last updated on 2013 Aug 30; Last cited on 2013 Oct 04].
- Wessberg J, Stambaugh CR, Kralik JD, Beck PD, Laubach M, Chapin JK, et al. Real-time prediction of hand trajectory by ensembles of cortical neurons in primates. Nature 2000;408:361-5.
- Velliste M, Perel S, Spalding MC, Whitford AS, Schwartz AB. Cortical control of a prosthetic arm for self-feeding. Nature 2008;453:1098-101.
- Wikipedia Free Encyclopaedia [www.wikipedia.org]. The encyclopedia. Available from: http://www.en.wikipedia. org/wiki/Artificial_neural_network. [Last updated on 2013 Oct 02; Last cited on 2013 Oct 04].
- Zeck G, Fromherz P. Noninvasive neuroelectronic interfacing with synaptically connected snail neurons immobilized on a semiconductor chip. Proc Natl Acad Sci U S A 2001;98:10457-62.
- Vogel G. Part man, part computer: Researcher tests the limits. Science 2002;295:1020.
- Wolpaw JR, Birbaumer N, Heetderks WJ, McFarland DJ, Peckham PH, Schalk G, et al. Brain-computer interface technology: A review of the first international meeting. IEEE Trans Rehabil Eng 2000;8:164-73.
- Hochberg LR, Serruya MD, Friehs GM, Mukand JA, Saleh M, Caplan AH, et al. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature 2006;442:164-71.
- Brain actuated technologies [www.brainfingers.com].Ohio: Brainfingers; c2013. Available from: http://www. brainfingers.com. [Last updated on 2013 Aug 28; Last cited on 2013 Oct 04].
- Haggard P, Kitadono K, Press C, Taylor-Clarke M. The brain’s fingers and hands. Exp Brain Res 2006;172:94-102.
- Herman N. Investigating the Use of Brainfingers in Children with Cerebral Palsy for Spatial-Motor Education. CA United States: Department of Mathematics, Harvey Mudd College; 2007.
- Coming soon: Artificial limbs controlled by thoughts. [www. scientificamerican.com]. United States: Scientific American; c2013. Available from: http://www.scientificamerican.com/ article.cfm?id=artificial-limbs-controlled-by-thought [Last updated on 2013 Oct 30; Last cited on 2013 Nov 7].




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.