Purpura Fulminans with Disseminated Intravascular Coagulopathy and Symmetric Peripheral Gangrene Complicating Sepsis in an Infant: A Case Report
Citation: Umar LW, Ya’uba MS, Olorukooba AA, Abubakar Y,Mohammed AJ, Chom ND. Purpura Fulminans with Disseminated Intravascular Coagulopathy and Symmetric Peripheral Gangrene Complicating Sepsis in an Infant: A Case Report. Ann Med Health Sci Res. 2017. 7:69-72
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Purpura fulminans is a rare consequence of sepsis that could be complicated by DIC and gangrene. We report the case of a 2-months-old infant who developed gangrenous limbs. He presented with fever, diarrhoea, vomiting and irritability for 5 days and was dehydrated and unconscious with global hypertonia. With a clinical impression of sepsis antibiotics, IV fluids and supportive care were commenced. He developed purpuric rashes over the trunk and feet, the latter of which gradually ascended over the legs, with swellings and ischemic skin changes up to the knees. A diagnosis of purpura fulminans was made and further evaluation revealed deranged haematologic parameters, features of Disseminated Intravascular Coagulopathy (DIC), hypernatraemia, azotaemia and arterial occlusion. Despite blood transfusions and supportive care gangrene emerged and progressively ascended towards the knees. Parents however rejected offer for surgical amputation and left against medical advice. The coexistence of purpura fulminans with multiple triggers for DIC should alert clinicians of a potential risk of peripheral gangrene.
Keywords
Infant, Sepsis, Purpura fulminans, DIC, Gangrene
Introduction
Purpura fulminans (PF) is characterized by cutaneous haemorrhage and necrosis caused by disseminated intravascular coagulopathy (DIC) and dermal small vessel thrombosis. It is commoner in infants and younger children, and often associated with multi-organ failure and death. The three categories of PF include the “inherited or acquired abnormalities of protein C or other coagulation proteins”, the “acute infectious PF” and the “idiopathic PF”.[1,2] It may occur in meningococcal infection, as with streptococcal, staphylococcal, gram-negative sepsis and Falciparum malaria.[2] Reports with Klebsiella isolates associated with PF are rather scanty, but Klebsiella rhinoscleromatis was reported in a three year-old Nigerian child with PF, DIC and symmetric gangrene and auto-amputation of the toes.[3]
The acute inflammatory response in sepsis activates coagulation and complement cascades leading to endothelial injury, which triggers DIC. The consumption of clotting factors and platelets leads to bleeding, while loss of key anticoagulants, Protein C and co-factor Protein S, promote thrombosis, inhibit fibrinolysis and further activates inflammation.[4] The lesions, which are less distinct on the black skin, are uniformly distributed over the lower half of the body in both the idiopathic and the proteins C and S deficiency sub-types. In contrast, infectious PF begins distally with proximal progression and diffuse spread, sometimes involving muscle and bone.[1] Ischaemia results from minor vascular obstruction but major vessel occlusions have been reported.[5] Tissue necrosis could involve bilaterally symmetrical sites and progress proximally, a condition referred to as symmetric peripheral gangrene.[6]
Infectious PF is triggered by endotoxin and widespread purpura has prognostic significance, especially in meningococcemia. The histopathologic features of infectious PF occur due to discrepancy between anticoagulant and pro-coagulant activities of endothelia. Although triggered by endotoxin, these disturbances are mediated by cytokines, notably interleukin-12, interferon-7, tumor necrosis factor and interleukin-1, leading to consumption of proteins C, S and anti-thrombin III.[7] Hypernatraemic dehydration also causes hypoperfusion and hyper viscosity of blood with subsequent ischaemia.[8]
PF may lead to auto or surgical amputation in up to 50% of survivors and a mortality of up to 52%.[6,9] Although the pathology once triggered is irreversible, prompt identification of potential risk factors with early haematologic evaluation provides opportunity for timely institution of appropriate management to reduce mortality and prevent debilitating tissue damage.[2,6-9] A study of potential triggers of coagulopathy associated with DIC among ill children found that 95.5% had thrombocytopenia, 93.4% had schistocytes, 74% had increased fibrin degradation products (FDPs) and 71.9% had prolonged clotting before purpura or features of DIC appeared.[9] The case reported here highlights the need for high index of suspicion in infants presenting with sepsis along with other risk factors for PF and DIC. The parents of this infant gave verbal consent for publication and other teaching purposes using the child’s clinical information on condition of ensuring anonymity of child’s identity.
Case History
S.B, a 2-month-old infant presented with fever, diarrhoea, vomiting and excessive crying for 5 days. He had passed only scanty urine twice within 24 hours before presentation, with reduced frequency and duration of breastfeeding. Un-prescribed syrups and poorly home-constituted ORS administered were ineffective and he became unresponsive four hours before presentation. He was still unconscious on examination with Blantyre score 2/5, temperature of 38.0°C, with pallor, severe dehydration and delayed capillary refill. He was tachypnoec (58 cycles/min) but with normal other respiratory findings. He had tachycardia (150/min) with feeble pulses and a blood pressure of 65/30 mmHg with normal other cardiovascular findings. He had hypertonia and hyper-reflexia but no other abnormal findings.
An impression of severe sepsis and shock was made, but the haemolytic uremic syndrome (HUS) and thrombotic thrombocytopenic purpura (TTP) were also considered as differential diagnoses. He was resuscitated with Normal saline infusion 20 ml/kg over one hour, repeated after poor response, followed by half-strength Darrow’s solution 80 ml/kg/24 hours plus replacement of on-going and insensible losses. He also had parenteral ceftriaxone with other supportive management.
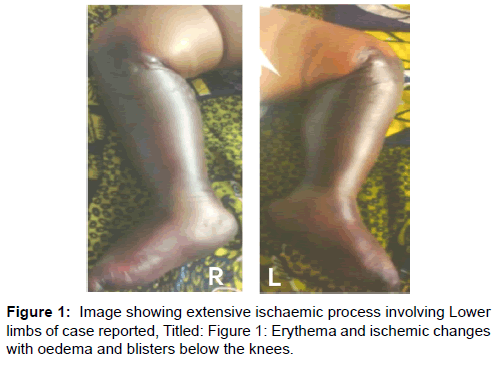
His condition gradually stabilized, evidenced by adequate urine output and improved vital signs, while the diarrhoea stopped by the third day when he regained consciousness and was breastfeeding, even though still irritable with intermittent inconsolable cry. A crop of erythematous macules and ecchymotic patches began to appear over the soles and the legs symmetrically, associated with tenderness and non-pitting oedema. The proximal margin of the lesion was demarcated from proximal healthy tissue by a zone of blisters lined circumferentially at the level of the tibial tuberosity. The popliteal and dorsalis pedis pulsations were not palpable on either side while other pulses were normal. The erythematous lesions gradually merged with adjacent healthy areas within 24 hours [Figure 1].
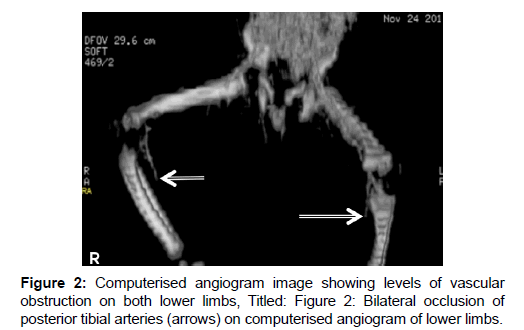
Prolonged bleeding was observed following injections and venepunctures and investigations then [Table 1] revealed abnormalities supportive of severe sepsis (immature forms of leukocytes, nucleated and deformed red blood cells [RBCs], thrombocytopenia), features of DIC (schistocytes, thrombocytopenia, deranged clotting parameters) and biochemical derangements suggestive of renal failure. Further evidence for DIC (plasma D-dimer, fibrinogen level and urinary fibrinogen degradation products [FDPs]) was unavailable due to lack of assay facilities. Blood culture isolated Klebsiella pneumoniae sensitive to ceftriaxone, among others. Intravenous metronidazole and pentazocin were added to the treatment upon appearance of the erythematous lesions. A double volume exchange blood transfusion was performed, followed by two whole blood transfusions over 72 hours, in spite of which a gangrenous process gradually appeared, ascending from the feet towards the knees. The overlying skin became darker and colder and the limbs became immobile with loss of pain and touch sensation, along with change in consistency to hard and dry. Computerized angiography showed normal outline of common iliac and femoral arteries but a gradual narrowing and obliteration of the lumen of both posterior tibial arteries below the knee joint bilaterally [Figure 2].
| Investigation Results | ||
|---|---|---|
| FBC | Blood film | Clotting profile |
| •PCV: 0.35 l/l •Total WBC: 12.9 × 109/L •Neutrophils 43%; •Lymphocytes 32%; •Bands 21%; •Platelets: 51 × 109/L. |
RBCs: •Aniso-poikilocytosis; •Boat shaped cells; •Cremated cells; •Schistocytes; •Nucleated RBCs 20/100 WBCs: •Left shift •Atypical lymphocytes •Cytoplasmic vacuolation |
•Prothrombin time (PT): 108 sec (Control 14 sec) •Activated partial thrombin time (APTT): >2 minutes (Control 32 sec) |
| Serum E/U (mmol/l) | Blood Culture | CSF |
| •Urea: 29.5 •Na+: 178 •Cl-: 120 •HCO3-: 20 •K+: 4.5 •Creatinine: 177 •Random blood glucose 9.3 mmol/L |
•Culture: Growth of Klebsiella pneumoniae •Sensitive to Ceftriazone, Cotrimoxazole, Colistin sulphate, •Gentamicin •Resistant to Chloramphenicol, Amoxycillin |
•Gross: Clear •Cell count <5 × 106 /mm3 •Protein - 211 mg/L; •Glucose - 6.3 mmol/L |
Table 1: Laboratory investigations showing deranged haematologic, clotting and biochemical profiles of case (S.B.)
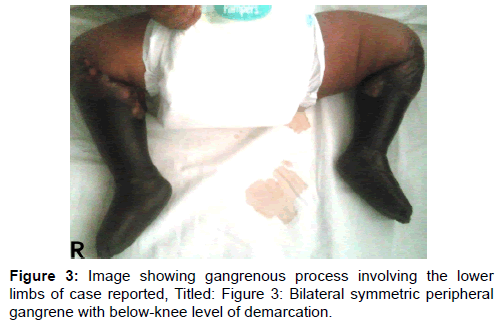
Fresh frozen plasma was considered for use but it was not readily available, while anti-coagulation with heparin also considered, could not be used due to bleeding that had already manifested in this patient. The gangrene gradually progressed in spite of measures taken at this point [Figure 3], while invited review by orthopaedic surgical team led to an offer for a bilateral above-knee amputation soon as clear line of demarcation of the gangrenous process became distinct. The parents however, unfortunately rejected this management option, while several counselling sessions carried out thereafter were not helpful and they eventually took the infant away for alternative care at home.
hypernatraemia in this infant, each of which is a potent predisposing factor for purpura fulminans and DIC, contributed to development of gangrene. Although PF is more commonly associated with meningococcemia, [2,7,10] Klebsiella species, found in this case, has previously been reported as a trigger for PF.[3] The progression of ischaemic gangrene is nearly consistent with most descriptions of infectious PF.[2,7] The arterial occlusions observed contrasts with the commoner micro-vascular thrombosis, which makes this case rather atypical. However, angiography has previously demonstrated similar tibial arterial occlusions at mid-calf, albeit unilateral, in an adult with PF, who survived following above-knee surgical amputation.[5] The haematologic and coagulation derangements along with thrombocytopenia observed suggested DIC. However, the lack of Protein C, Protein S, Anti-thrombin III, plasma and urinary FDPs assays constituted major limitations to completeness of laboratory evaluation in this infant, especially as these parameters are useful for confirmation and staging of DIC.[2,3,7,9]
The empirical choice of ceftriaxone was coincidentally appropriate (as per antibiotic sensitivity) for the isolated organism, to which there was objective clinical response. In spite of this along with fluid replacements and multiple blood transfusions the gangrenous process advanced unabated. The lack of facilities for platelet, coagulation factor, Protein C, Protein S, Antithrombin III concentrates, Fresh frozen plasma or cryoprecipitate known to be beneficial in patients with PF and DIC,[2,7] constituted further management limitations. This informed our resort to use repeated fresh blood transfusions after the initial exchange transfusion aimed to reduce pro-inflammatory mediators elaborated in sepsis.[3] Poor response of gangrenous process has similarly been observed in previous reports that mostly documented reduction of mortality without influence on gangrene progression.[1-3,6,10] We do not have information on outcome because hospital management was prematurely terminated. Surgical amputation involves tissue debridement, skin grafting and bony amputation of affected limbs.[6] Fluid replacement is paramount to restore lost volumes, reverse renal failure and maintain adequate urine output. [7,8] Hypernatraemia has been recognized as a potent risk factor for the development of purpura fulminans and DIC.[8] The infant was severely dehydrated and had features of circulatory collapse (shock) at presentation. Further laboratory evaluation revealed serum Na+ of 178 mmol/dl and Cl- of 120 mmol/dl, consistent with severe hypernatraemic dehydration. Although these findings were prevailing at presentation, normal saline was still used for resuscitation because it is one of the fluids recommended as a plasma expander in the management of septic or hypovolemic shock (including that due to hypernatraemia). The volumes of normal saline and Half-strength Darrow’s solution used for this infant to correct shock and dehydration were within recommended limits and were unlikely to have worsened the already elevated serum concentrations of Na+ or Cl-.
The use of heparin for PF with DIC is not supported by evidence as it may aggravate thrombocytopenia and haemorrhage.[3] Platelet concentrate infusions (for counts below 20,000/mL) may reduce morbidity and mortality.[2,7,11]
The differential diagnoses considered, even though also rare, included HUS and TTP, are also characterised by microangiopathy, intravascular haemolysis, thrombocytopenia and renal failure.[3,11,12] The pathological hallmark in HUS occurs in the kidney leads to haemolytic anemia and ischemic injury. Its commonest form associated with gastrointestinal infection by Shiga toxin-producing Escherichia coli, has better prognosis compared to the rarer atypical form which progresses to end stage renal disease in 50% of cases with 15% mortality.[1,12] This infant had acute diarrhoea, a feature seen with both Shiga toxin producing Eschericia coli and of Klebsiella, also capable of endotoxin elaboration. Also, while peripheral gangrene complicating atypical HUS was previously reported,[12] symmetric distribution, ecchymosis, prolonged prothrombin time (PT) and activated partial thrombin time (APTT) are uncommon in HUS and TTP.[3] We could not rule out genetic deficiency of Proteins C, S or Anti-thrombin III resulting in complement-mediated endothelial damage in this patient, and thus, the interplay of multiple risk factors identified may have simply served as triggers interacting in unison.
It was an ethical dilemma for the managing team regarding the parents’ decision to opt for discharge against medical advice. This gravely ill infant lacked the emotional and cognitive maturity required for decision-making, and was thus dependent on his parents’ prerogative for choice of healthcare to treat his ailment. Bound by the Hippocratic oath,[13] to uphold the welfare of this infant, we were caught between acting in the infant’s best interest (beneficence) and allowing the parents’ decision to prevail. Although counseling was done on the need for continuing hospital care and made clear the likely untoward consequences of terminating care prematurely, all was to no avail. It was nevertheless, also made clear the team’s readiness to re-admit the child in the event the parents reconsidered returning after leaving hospital. Based on a spiritual conviction on prayer as alternative and lack of resources to cater for costs of surgical care the parents opted for discharge against medical advice. This infant could have benefitted from the national health insurance scheme that is being operated in the facility, but its national coverage of only 3%,[14] (as at the time this infant was managed) was still limited to hospital and other government employees. We do not have legal rights to detain children in such circumstances, especially as the surgical care being offered was unaffordable by these poor parents and we could not influence their religious conviction regarding the effectiveness of treatment options.
Conclusion
The concurrence of multiple risk factors for purpura fulminans and DIC should alert clinicians to a risk of development of symmetric peripheral gangrene. Early recognition and prompt management are crucial to reduce morbidity and mortality.
REFERENCES
- Piparsania S, Rajput N, Singh K. Purpura fulminans in infantile streptococcal septicemia. Surgical Sci.2011;2:496-498.
- Chalmers E, Cooper P, Forman K. Purpura fulminans: recognition, diagnosis and management. Arch Dis Child.2011;96:1066-1071.
- Olowu WA. Klebsiella-induced purpura fulminans in a Nigerian child: Case report and a review of literature. WAJM. 2002;21:253-256.
- Páramo JA, Pérez JL, Serrano M. Types 1 and 2 plasminogen activator inhibitor and tumor necrosis factor alpha in patients with sepsis. Thromb Haemost.1990;64:3-6.
- Jaconelli T, Psirides A. A fulminant rash. New Z Med J.2009;122:73-74.
- Ghosh SK, Bandyopadhyay D. Symmetrical peripheral gangrene. Ind J Dermatol VenereolLeprol.2011;77:244-248.
- Darmstadt GL. Acute infectious purpura fulminans: Pathogenesis and medical management. Pediatr Dermatol.1998;15:169-183.
- Amitai I, Goder K, Husseini N. Hypernatremic dehydration complicated by peripheral gangrene in infancy. Isr J Med Sci. 1983;6:538-540.
- Chuansumrit A, Hotrakitya S, Hathirat P. Disseminated intravascular coagulation in children: diagnosis, management and outcome. Southeast Asian J Trop Med Public Health. 1993;1:229-233.
- Akar M, Demirel FG, Sandal G. Neonatal Purpura Fulminans: A report of seven case. Turkish J Pediatr Dis.2011;5:95-99.
- Nolan J, Sinclair R. Review of management of purpura fulminans and two case reports.Brit J Anaesth.2001;86:581-586.
- Malina M, Gulati A, Bagga A. Peripheral gangrene in children with atypical hemolytic uremic syndrome. Pediatrics. 2013;131:331-335.
- World Medical Association. Medical Ethics Manual 2005. Available at: http://www.wma.net/en/30publications/30ethicsmanual/pdf/Ethics_manual_3rd_Nov2015_en.pdf. Accessed 5 November 2016.
- Arin D, Hongoro C. Scaling up national health insurance in Nigeria: Learning from case studies of India, Colombia, and Thailand. Washington, DC: Futures Group, Health Policy Project. 2013. Available at: http://www.healthpolicyproject.com/pubs/96_NigeriaInsuranceFinal.pdf. Accessed 4 November, 2016.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.