Quantification and Localization of Mast Cells in Periapical Lesions
- *Corresponding Author:
- Dr. Manjunatha BS
Department of Dental Anatomy and Oral Biology, Basic Dental Sciences, Faculty of Dentistry, Taif University, Al-Taif-21944, Kingdom of Saudi Arabia.
E-mail: drmanju26@hotmail.com
Abstract
Background: Periapical lesions occur in response to chronic irritation in periapical tissue, generally resulting from an infected root canal. Specific etiological agents of induction, participating cell population and growth factors associated with maintenance and resolution of periapical lesions are incompletely understood. Among the cells found in periapical lesions, mast cells have been implicated in the inflammatory mechanism. Aim: Quantifications and the possible role played by mast cells in the periapical granuloma and radicular cyst. Hence, this study is to emphasize the presence (localization) and quantification of mast cells in periapical granuloma and radicular cyst. Materials and Methods: A total of 30 cases and out of which 15 of periapical granuloma and 15 radicular cyst, each along with the case details from the previously diagnosed cases in the department of oral pathology were selected for the study. The gender distribution showed male 8 (53.3%) and females 7 (46.7%) in periapical granuloma cases and male 10 (66.7%) and females 5 (33.3%) in radicular cyst cases. The statistical analysis used was unpaired t‑test. Results: Mean mast cell count in periapical granuloma subepithelial and deeper connective tissue, was 12.40 (0.99%) and 7.13 (0.83%), respectively. The mean mast cell counts in subepithelial and deeper connective tissue of radicular cyst were 17.64 (1.59%) and 12.06 (1.33%) respectively, which was statistically significant. No statistical significant difference was noted among males and females. Conclusion: Mast cells were more in number in radicular cyst. Based on the concept that mast cells play a critical role in the induction of inflammation, it is logical to use therapeutic agents to alter mast cell function and secretion, to thwart inflammation at its earliest phases. These findings may suggest the possible role of mast cells in the pathogenesis of periapical lesions.
Keywords
Mast cells, Periapical granuloma, Radicular cyst, Toluidine blue
Introduction
Mast cell is resident of connective tissue and contains many granules rich in histamine and heparin. Although best known for their role in allergy and anaphylaxis, mast cell plays an important protective role as well, being intimately involved in wound healing and defense against pathogens.[1] Mast cells are present in most tissue in the vicinity of blood vessels and are especially prominent near skin, mucosa of the lungs, and digestive tract as well as in the Oral cavity, conjunctiva, and nose.[2]
Specific etiological agents of induction, participating cell population and growth factors associated with maintenance and resolution of periapical lesion are incompletely understood. Among the cells found in periapical lesions, mast cells have been detected in the inflammatory infiltrate of granulomas and cysts, suggesting a role for mast cells in the inflammatory mechanisms of these lesions.[3] There is increased awareness of the potential range of mast cell function and interactions in the immune response, including expanding understanding of the role of mast cell in modulating the immune response of humoral and cellular events.[4] It has become clear that mast cells are able to produce a wide range of mediators, creator linkages between early mast cell activation and subsequent T-lymphocytes stimulation in cell–mediated inflammatory diseases, including periapical lesions, inflammatory reactions driven by T cell–mast cell interaction can be expected to have degree of redundancy in terms of mediators.[5] Very few studies have been done on the quantifications and the possible role played by mast cells in the periapical granuloma and radicular cyst. Hence, this study was undertaken to emphasize the localization and quantification of mast cells in periapical granuloma and radicular cyst.
Materials and Methods
The present study consisted of a total 30 previously diagnosed cases of periapical cyst and periapical granuloma (15 cases each) were selected from the archives of the Department of Oral Pathology and Microbiology, K M Shah Dental College and Hospital, Vadodara. The buffered formalin-fixed paraffin-embedded tissues were taken along with the clinical details from the files. Two sections of 5 μm thick sections on different microscopic slides were taken from each block, and one slide was stained with routine hematoxylin and eosin stain for confirmation of clinical diagnosis and histological evaluation. The second set of slides was stained with the toluidine blue stain (1% toluidine blue in 1% sodium chloride) for enumeration of mast cells. The slides were observed under binocular research microscope under various magnifications.
Ethical considerations
Our research was conducted in full accordance with the World Medical Association Declaration of Helsinki. Prior to the beginning of the study, the study proposal was submitted, and approval was obtained from the Institutional Ethical Committee.
Evaluation of staining
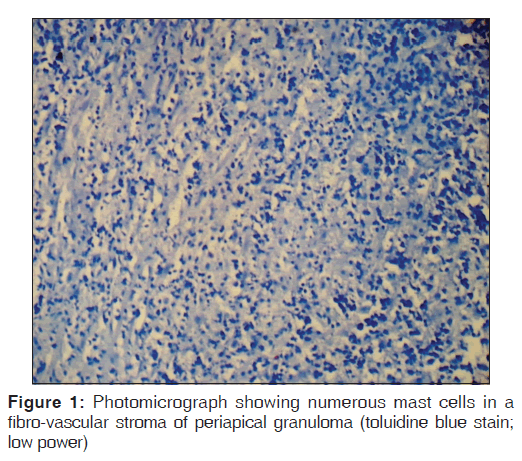
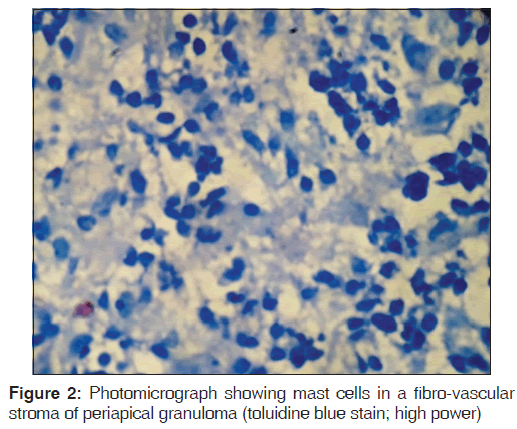
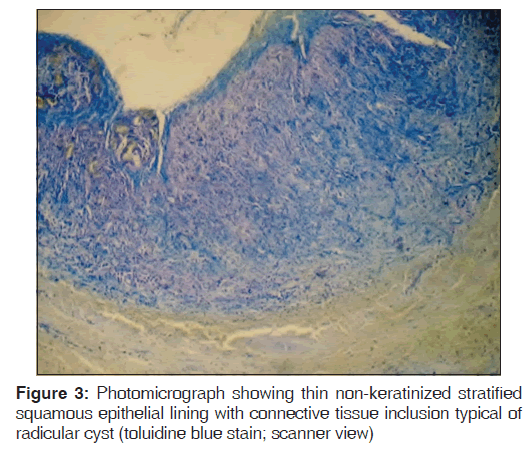
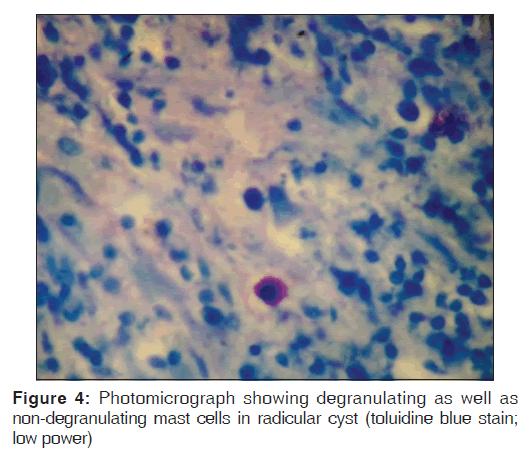
Toluidine blue stained sections of periapical granuloma and radicular cyst were analyzed under different magnifications, and five fields were selected from the entire connective tissue for mast cells counting [Figures 1-4]. Both intact [Figures 1 and 3] and degranulated mast cells [Figures 2 and 4] were recorded under ×40 magnification of the microscope for counting. Results were expressed as the average number of mast cells per high power field out of five fields.
Statistical analysis
All the data were compiled on Microsoft Excel software and processed by Statistical Package for Social Sciences, Windows 7, software version 14 (Integral Solutions Limited, IBM Company). Unpaired t-test was used, and all the comparisons were evaluated. The connective tissue just beneath the epithelium is taken as subepithelial and from the submucosal area we considered as deeper connective tissue.
Results
Table 1 shows a mean mast cell count of 12.40 (0.99) and 7.13 (0.83) subepithelially and deeper connective tissue in periapical granuloma cases. Statistically significant differences were observed between subepithelially and deeper connective tissue (P < 0.001). Mean is used as it is categorical data and data has no outliers. Table 2 shows a mean mast cell count of 17.64 (1.59) and 12.06 (1.33) subepithelially and deeper connective tissue in radicular cyst, statistically significant differences were observed between subepithelially and deeper connective tissue (P < 0.001). Table 3 shows mast cell count between periapical granuloma and radicular cyst in males and females. In the current study, 8/15 males (53.3%) and females 7/15 (46.7%) in periapical granuloma cases and 10/15 male (66.7%) and 5/15 females (33.3%) in radicular cyst cases, statistically no significant differences were observed (P < 0.5). Table 4 shows a statistically significant difference between mast cells in periapical granuloma and periapical cyst (P < 0.001).
| Site | n | Mean (SD) | P |
|---|---|---|---|
| Subepithelial | 15 | 12.40 (0.99) | <0.001 |
| Deeper connective tissue | 15 | 7.10 (0.83) | |
SD: Standard deviation
Table 1: Comparision of mast cell count in periapical granuloma between subepithelial and deeper connective tissue
| Site | n | Mean (SD) | P |
|---|---|---|---|
| Subepithelial | 15 | 17.64 (1.59) | <0.001 |
| Deeper connective tissue | 15 | 12.06 (1.33) | |
SD: Standard deviation
Table 2: Comparision of mast cell count in radicular cyst between subepithelial and deeper connective tissue
| Site | Male | Female | P |
|---|---|---|---|
| Periapical granuloma | 8 | 7 | 0.46 |
| Radicular cyst | 10 | 5 | |
SD: Standard deviation
Table 3: Comparision of mast cell count in periapical granuloma and radicular cyst between male and females
| Site | Subepithelial (mean (SD)) | Deeper connective (mean (SD)) | P |
|---|---|---|---|
| Periapical | 12.4 (0.99) | 7.13 (0.83) | |
| granuloma | 04304 | 380939 | <0.001 |
| Radicular cyst | 17.64 (1.59) | 12.06 (1.33) | |
| SD: Standard deviation |
SD: Standard deviation
Table 4: Comparision of mast cell count in periapical granuloma and radicular cyst between subepithelial and deeper connective tissue
Discussion
Mast cells were first discovered by Paul Ehrlich in his 1878 doctoral thesis on the basis of their unique staining characteristics and large granules. These granules also led him to the mistaken belief that existed to nourish the surrounding tissue, and he named them “mastzellen” meaning feeding cells. Nowadays, they are considered to be part of body immune system.[6]
Periapical lesions occur in response to chronic irritation in periapical tissue generally resulting from an infected root canal.[4] A great variety of bacterial antigens may stimulate host immune responses, including antigen-specific immunologic response and nonspecific inflammatory reactions. Specific etiological agents of induction, participating cell population and growth factors associated with maintenance and resolution of periapical lesions are incompletely understood.[7] Among the cells found in periapical lesions, mast cells have been detected in the inflammatory mechanisms of these lesions.[4] Radicular cysts are thought to be derived from the epithelial rests within periapical granuloma, the periapical granuloma being a common sequel of pulp necrosis in which bacterial antigens in the root canal may be potential stimulants for immunological reactions.[8] It was previously suggested that mast cell are active in chronic inflammatory periapical reactions. They reported that numerous mast cells were presents in their specimens, but cellular quantification was not made several studies reported the presence of mast cells in the periapical inflammatory lesions.[7] Perrini and Fonzi studied periapical granuloma and argued that mast cells were difficult to locate.[9]
In the present study, periapical granuloma and radicular cyst localization and the quantitative analysis of mast cells were done using toluidine blue stain. Counting of mast cells was done in two different zones namely subepithelial and deeper connective tissue in both lesions. Mast cell counts in five different fields were recorded under ×40 magnification of the microscope for counting. A mean count of mast cells is 12.4 and 7.13 in subepithelial and deeper connective tissue of periapical granuloma. The mean mast cell count in subepithelial and deeper connective tissue of radicular cyst is 17.64 and 12.06, respectively. In comparison to deeper areas of connective tissue, the subepithelial zone had more number of mast cells, and this was statistically significant (P < 0.001). The mast cell count was compared between male and females in both periapical granuloma and radicular cyst there was no statistical significant difference observed. The standard deviation is used in conjunction with the mean to summarize continuous data, not categorical data. In addition, the standard deviation, like the mean, is normally only appropriate when the continuous data are not significantly skewed or has outliers.
A recent study found a significant relationship between mast cell number and survival and grade of colorectal carcinoma. There was a correlation between mast cell number and microvessel density. The study suggested that mast cell number may be used as alternative criteria to the standard criteria used to determine the prognosis of colorectal carcinoma.[10]
These results are in accordance with the study conducted by Smith et al. in which they enumerated the presence of mast cells through the thickness of the cyst capsule indicated that they were more prevalent just beneath the epithelium than in deeper areas of cyst.[11] Some studies have shown subepithelial banding due to the presence of heparin from degranulated mast cells which probably help in the movement of inflammatory products through the epithelium and account for glycosaminoglycans in cyst fluids. This implies a chemotactic stimulus to mast cells in odontogenic cysts attract to the epithelial lining or luminal fluid contents. Mast cells also have been implicated in stimulating the production of prostaglandins to be important in bone resorption for odontogenic cyst growth.[8,11,12]
Periapical granuloma and cysts seem to develop from the inflammation in the dental pulp.[13] Immunological reactions against the bacteria and/or their products, host components altered by infection and/or against substance used in the treatment may help to maintain the inflammation and lead to the formation of granuloma and cyst.[4] The presence of mast cells in periapical chronic inflammatory lesions is shown by many authors and suggested that mast cells play an important role in the pathogenesis of chronic inflammatory lesions. In the present study, the number of mast cells may have been counted low due to limitation of toluidine blue staining which has low sensitivity to detecting partially degranulated mast cells.
Conclusion
Periapical granuloma and radicular cysts are recognized as one of the most common inflammatory periradicular reactions of pulpal disease. Radicular cyst derives inflammatory proliferation and cystic degeneration of epithelial rests of Malassez contained in periapical granulomatous tissue, usually secondary to pulp necrosis. Among the cells found in these periapical inflammatory lesions, mast cells have been detected in the inflammatory infiltrates of granulomas and cysts, suggesting a role in the inflammatory mechanisms of these lesions.
Based on the concept that mast cells play a critical role in the induction of inflammation, it is logical to use therapeutic agents to alter mast cell function and secretion, to thwart inflammation at its earliest phases. It may be possible to develop novel approaches that influence the release of pro-inflammatory molecules or neuropeptides to ameliorate mast cell-driven inflammation.
The authors think that it is difficult to determine the role of mast cells and their products in the expansion of the cystic lesions.
Source of Support
Nil.
Conflict of Interest
None declared.
References
- Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev 1997;77:1033-79.
- Sudhakar R, Ramesh V, Balamurali PD, Oza N, Premalatha B,
- Karthikshree V. Incidence of mast cells in oral inflammatory lesions a pilot study. J Oral Maxillofac Pathol 2005;9:12-5.
- Skaug N, Johannessen AC, Matre R, Nilsen R. In situ characterization of cell infiltrates in human dental periapical granulomas 2. Demonstration of receptors for the complement components C3b and C3d. J Oral Pathol 1984;13:111-9.
- Kabashima H, Nagata K, Maeda K, Iijima T. Involvement of substance P, mast cells, TNF-alpha and ICAM-1 in the infiltration of inflammatory cells in human periapical granulomas. J Oral Pathol Med 2002;31:175-80.
- Zhao ZZ, Savage NW, Walsh LJ. Associations between mast cells and laminin in oral lichen planus. J Oral Pathol Med 1998;27:163-7.
- Benoist C, Mathis D. Mast cells in autoimmune disease. Nature 2002;420:875-8.
- Nilsen R, Johannessen AC, Skaug N, Matre R. In situ characterization of mononuclear cells in human dental periapical inflammatory lesions using monoclonal antibodies.
- Oral Surg Oral Med Oral Pathol 1984;58:160-5.
- Teronen O, Hietanen J, Lindqvist C, Salo T, Sorsa T, Eklund KK, et al. Mast cell-derived tryptase in odontogenic cysts. J Oral Pathol Med 1996;25:376-81.
- Perrini N, Fonzi L. Mast cells in human periapical lesions: Ultrastructural aspects and their possible physiopathological implications. J Endod 1985;11:197-202.
- Elezoglu B, Tolunay S. The relationship between the stromal mast cell number, microvessel density, c-erbB-2 staining and survival and prognostic factors in colorectal carcinoma. Turk Patoloji Derg 2012;28:110-8.
- Smith G, Smith AJ, Basu MK. Mast cells in human odontogenic cysts. J Oral Pathol Med 1989;18:274-8.
- Landini G. Quantitative analysis of the epithelial lining architecture in radicular cysts and odontogenic keratocysts. Head Face Med 2006;2:4.
- Ledesma-Montes C, Garcés-Ortíz M, Rosales-García G, Hernández-Guerrero JC. Importance of mast cells in human periapical inflammatory lesions. J Endod 2004;30:855-9.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.