Rare Phenomenon of Psedodominance Causing Phenotypic Heterogeneity in the Expression of Delta Beta Thalassemia when Co-Inherited with Beta Mutation
Received: 03-Jan-2023, Manuscript No. amhsr-23-87809; Editor assigned: 05-Jan-2023, Pre QC No. amhsr-23-87809 (PQ); Reviewed: 20-Jan-2023 QC No. amhsr-23-87809; Revised: 27-Jan-2023, Manuscript No. amhsr-23-87809 (R); Published: 06-Feb-2023
Citation: Padma G, et al. Rare Phenomenon of Psedodominance Causing Phenotypic Heterogeneity in the Expression of Delta Beta Thalassemia when Co-Inherited with Beta Mutation. Ann Med Health Sci Res. 2023;13:420-424
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Delta beta thalassemia is a rare form of thalassemia characterized by complex pattern of phenotypes with varying degrees of severity depending on the gene mutation involved. Hence, accurate diagnosis of the patient becomes utmost important for proper treatment and genetic counselling to the patients. Four patients (3<1 yr and 1-4 yrs) initially diagnosed as thalassemia major based on their HPLC were reinvestigated based on parents HPLC (HbF<20% in one of the parents) by molecular screening using sequencing and Gap PCR. Sequencing of all the patients for HBB gene revealed homozygosity for c.92+5G>C (IVS1-5(G>C)) mutation in three patients and c.315+1G>A in one patient. All showed heterozygosity for Asian Indian Inversion deletion ((Gγ(Aγδβ)0) when tested for delta beta deletions. One of the parents showed carrier status for beta globin mutation and the other for Gγ(Aγδβ)0 deletion. Interestingly, patients with c.92+5G>C presented early onset with low HbF as compared to the patient with c.315+1G>A, showing phenotypic diversity depending on the nature of beta mutation. Finally, patients were diagnosed as compound heterozygous for Gγ(Aγδβ)0 and beta mutation (c.92+5G>C or c.315+1G>A) representing a rare pattern of pseudo-dominance leading to wrong interpretation of the genotype of the patient and indicating the necessity of considering parents HPLC before concluding molecular diagnosis.
Keywords
Delta-beta Thalassemia; Beta globin gene mutations; Pseudo-dominance; Fetal hemoglobin; diagnosis
Introduction
Thalassemia’s are a group of genetic blood disorders arising due to defective globin proteins. Depending on the genes involved in production of a defective globin protein thalassemia can be alpha, beta, or delta thalassemia. Delta beta thalassemia is a rare form of beta thalassemia characterized by decreased or absence of delta (δ) and beta (β) globin chains. It is found in different ethnic groups and around nine mutations have been reported to be causing the condition [1,2]. Delta beta thalassemia is also known as F-thalassemia, β thalassemia type 2 and normal A2 β-thalassemia [3]. It results from the deletion of delta and beta genes and as a compensatory mechanism gamma (γ) chain synthesis is increased resulting in elevated levels of fetal hemoglobin HbF in blood which is homogenously distributed in red blood cells. In homozygous delta beta cases the alpha/ non alpha chain imbalance is very less due to high HbF (100%) production because of which the patients show thalassemia intermedia like phenotype or a silent phenotype mostly requiring no transfusions but red blood cells showing high reticulocyte count with hypochromic microcytic morphology. Patients who are heterozygous for the condition tend to have a modest elevation of HbF (5-20%) with hypochromic microcytic red cell indices [4]. They have mild anemia similar to beta thalassemia trait and are considered as delta-beta thalassemia carriers [5,6]. In some cases, delta beta mutation is co-inherited with a beta- globin mutation exhibiting a complex pattern of phenotypes with varying degrees of severity depending upon the beta globin mutation involved. They present a clinical picture similar to thalassemia major and require blood transfusions. Hereditary Persistence of Fetal Hemoglobin (HPFH) is another condition in which patients show high levels of HbF (up to 30%) after infancy period similar to delta beta thalassemia and exhibit mild normochromic anemia. Due to similarities in the clinical features of delta beta thalassemia with other conditions it becomes utmost important to diagnose the condition accurately in order to provide an appropriate treatment and genetic counselling to the patients.
In the present study, we aim to describe the diagnosis of delta beta thalassemia with co-inheritance of beta mutation in four of the cases who were found to be compound heterozygous for Asian Indian Inversion deletion and a mutation in beta gene exhibiting a rare phenomenon of pseudo dominance wherein the recessive heterozygous mutation in beta gene mimics dominant inheritance pattern.
Materials and Methods
Kamala Hospital and Research Centre for Thalassemia and Sickle cell patients–A unit of Thalassemia and Sickle Cell Society (TSCS), Hyderabad, India is a not for profit organization providing diagnosis, treatment and counselling to thalassemia and Sickle cell anemia patients.
Of the patients registered at TSCS, Hyderabad, India, four patients were preliminarily diagnosed as thalassemia major based on the High Performance Liquid Chromatography (HPLC) results requiring frequent blood transfusions. However, when the HPLC data of the parents was observed, it was found that one of the parents had high fetal hemoglobin levels (HbF<20%) suggesting possible involvement of delta gene. Hence, these four cases were re-evaluated for molecular screening in beta and delta genes with informed consent from all the subjects who participated in the present study which was approved by institutional ethical committee. For molecular evaluation 2-3 ml of venous blood sample was collected in EDTA vacutainer from all the four patients and their parents to carry out micro profiling. DNA was isolated from the blood samples by salting out method and quantified using Nano spectrometer (ND-1000). Beta globin gene was amplified and screened for mutations causing beta thalassemia using automated sanger sequencing. GAP PCR was performed using deletion specific primers (Table 1) as per the protocol described by Craig et al., to detect the Asian Indian Inversion deletion Gγ(Aγδβ)0 in all the samples[7].
| Gene | Amplified Region | Primer sequence | Gene bank co-ordinates | Product length (bp) | A.T | |
|---|---|---|---|---|---|---|
| Delta-beta | 5’ Deletion | P1: 5' atg cca taa agc acc tgg atg 3' | 39827-39847 | N: 1195 | 56℃ | |
| P2: 5'gag ctg aag aaa atc atg tgt ga 3' | 41022-41000 | M: 327 | ||||
| P3: 5’taa cca tat gca tgt att gcc 3’ | 56110-56132 | |||||
| 3’ Deletion | P2: 5'gag ctg aag aaa atc atg tgt ga 3' | 41022-41000 | N: 665 | |||
| P4: 5’caa tgt atc atg cct ctt tgc ac-3’ | 63242-63264 | M: 371 | ||||
| P5: 5’gca gcc tca cct tct ttc atg g3’ | 63907-63886 | |||||
| HBB | Region1 | F: 5' gcc aag gac agg tac ggc tgt cat c 3' | 2785-2809 | 706bp | 57℃ | |
| R: 5' ccc ttc cta tga cat gaa ctt aac cat 3' | ||||||
| 3464-3490 | ||||||
| Region 2 | F: 5' gtg tac aca tat tga cca aat c 3' | 3876-3897 | 719bp | |||
| R: 5' aat gca ctg acc tcc cac at 3' | 4575-4595 |
Table 1: Primers used to identify the delta beta deletion and mutations in HBB gene.
Abbreviation: A.T: Annealing Temperature; N: Normal; M: Mutant
For GAP PCR two separate reaction mixtures were prepared to amplify the 5’ and 3’ regions of deletion fragment. PCR was performed in 20 μl of reaction volume constituted by adding 50 ng of genomic DNA, 1X PCR buffer, 200 μM dNTPs each, 2.5 pmoles of each primer and 0.25 units of Taq DNA polymerase. The PCR conditions used for amplifying both the regions were initial denaturation at 94℃ for 3 min followed by 30 cycles of denaturation at 94℃ for 1 min, annealing at 56℃ for 1 min and extension at 72℃ for 2 min and a final extension of 72℃ for 7 min. PCR products generated were genotyped on 1.5% agarose gels.
To identify the variants in HBB gene two sets of primers were used to cover the entire beta globin gene (Table 1). PCR was performed in 25 μl reaction volume prepared as stated above and the conditions used to amplify both the regions were 95℃ for 5 min followed by 35 cycles of denaturation at 95℃ for 45 sec, annealing 57℃ for 30 secs, extension 72℃ for 35 sec and ended up with a final extension of 72℃ for 5 min. Amplification of the PCR products was checked on 1% agarose gel and amplified products were sequenced to identify the nature of the mutation.
Results
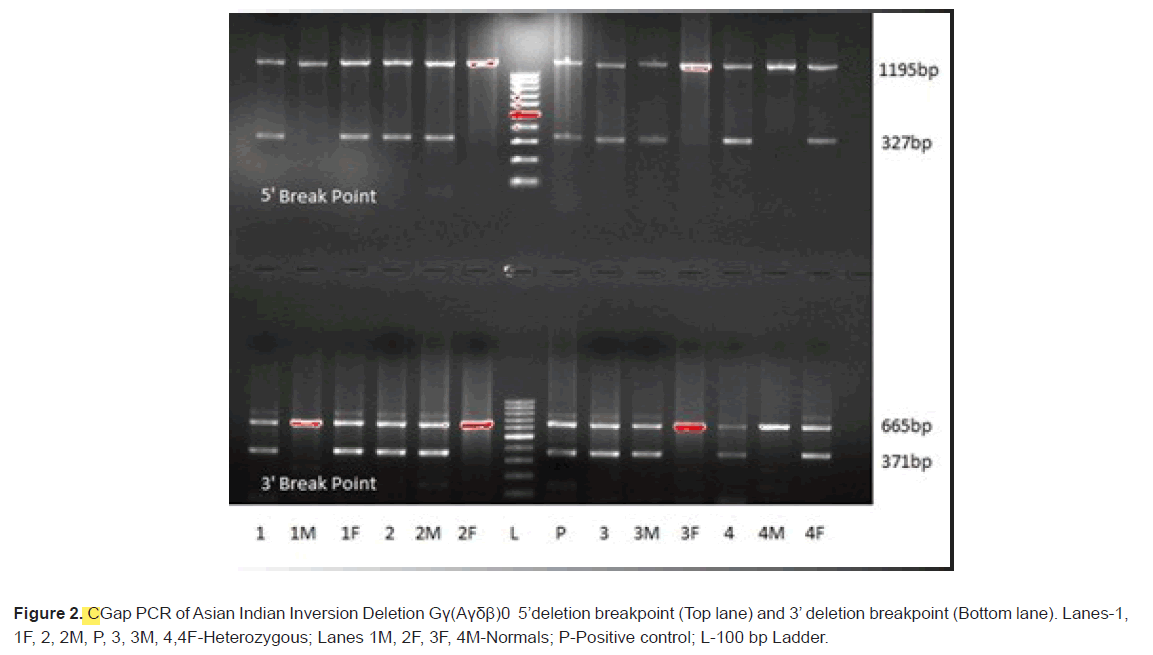
A total of four patients who were initially considered as thalassemia major based on HPLC results were analysed for delta beta thalassemia after examining the HPLC data of the parents. Red blood cells of the patients showed abnormal distribution and were hypochromic, microcytic with slight anisocytosis. Three patients were diagnosed below the age of 1 year while one patient showed late age at onset (after 4 yrs; Table 2). All the four patients required transfusions and behaved like thalassemia major. Initial screening of the four patients for mutations in beta globin gene through sequencing revealed homozygosity for c.92+5G>C mutation in three patients and one patient showed homozygosity for c.315+1G>A mutation (Figure 1). However, when the parent’s samples were analysed for hemoglobin levels (HbA, HbA2 and HbF) through HPLC, it was found that one of the parents showed high fetal hemoglobin levels indicating the involvement of delta gene mutation or Hereditary Persistence of Fetal Hemoglobin (HPFH). Hence, all the four patients were also analysed for the most common Asian Indian Inversion deletion in delta gene causing delta beta thalassemia and Asian specific HPFH-6 and HPFH-3 deletion. All of them showed heterozygosity for Asian Indian Inversion deletion while they were normal for HPFH-6 and HPFH-3. Further analysis of the parents of the four patients for mutations in beta globin gene by sequencing revealed that only one of the parent was a carrier for a mutation in beta globin gene (c.92+5G>C in three and c.315+1G>A in one patients; Figure 1). Hence, the other parents were analysed for Asian Indian Inversion deletion by Gap PCR and were found to be carriers (Figure 2). This suggests that though the patient shows homozygosity for beta mutation on sequencing, they are actually compound heterozygotes with beta mutation being inherited from one parent and deletion from the other parent. In genetics, this represents a case of pseudodominance.
| Parameter | Case 1 | Case 2 | Case 3 | Case 4 |
|---|---|---|---|---|
| Age | 6 yrs | 3 yrs | 12 yrs | 3 yrs |
| Age at Diagnosis | 5 mnths | 9 mnths | 4 yrs | 18 mnths |
| Age at First transfusions | 5 mnths | 9 mnths | 5 yrs 9 mnths | 18 mnths |
| Total Transfusion | 49 | 14 | 69 | 7 |
| Hb (gms/dl) | 5.6 | 6.9 | 7.5 | s9.2 |
| RBC *106 / | 2.7 | 2.5 | 4.3 | 3.93 |
| MCV (fl) | 70.4 | 67 | 70.1 | 73.3 |
| MCH (pg) | 20.7 | 27 | 23.3 | 23.4 |
| RDW (%) | 30.2 | 20 | 33 | 30.9 |
| RBC Morphology | Abnormal Distribution | Microcytosis, Hypochromia | Microcytosis, Hypochromia | Microcytosis, Hypochromia |
| Anisocytosis | ||||
| HB A2 | 0.9 | 2 | 0.4 | 1.5 |
| HB F | 78.8 | 79.5 | 96.7 | 76.7 |
Table 2: Baseline characteristic features and hematological data of patients.
A total of four patients who were initially considered as thalassemia major based on HPLC results were analysed for delta beta thalassemia after examining the HPLC data of the parents. Red blood cells of the patients showed abnormal distribution and were hypochromic, microcytic with slight anisocytosis. Three patients were diagnosed below the age of 1 year while one patient showed late age at onset (after 4 yrs; Table 2). All the four patients required transfusions and behaved like thalassemia major. Initial screening of the four patients for mutations in beta globin gene through sequencing revealed homozygosity for c.92+5G>C mutation in three patients and one patient showed homozygosity for c.315+1G>A mutation (Figure 1). However, when the parent’s samples were analysed for hemoglobin levels (HbA, HbA2 and HbF) through HPLC, it was found that one of the parents showed high fetal hemoglobin levels indicating the involvement of delta gene mutation or Hereditary Persistence of Fetal Hemoglobin (HPFH). Hence, all the four patients were also analysed for the most common Asian Indian Inversion deletion in delta gene causing delta beta thalassemia and Asian specific HPFH-6 and HPFH-3 deletion. All of them showed heterozygosity for Asian Indian Inversion deletion while they were normal for HPFH-6 and HPFH-3. Further analysis of the parents of the four patients for mutations in beta globin gene by sequencing revealed that only one of the parent was a carrier for a mutation in beta globin gene (c.92+5G>C in three and c.315+1G>A in one patients; Figure 1). Hence, the other parents were analysed for Asian Indian Inversion deletion by Gap PCR and were found to be carriers (Figure 2). This suggests that though the patient shows homozygosity for beta mutation on sequencing, they are actually compound heterozygotes with beta mutation being inherited from one parent and deletion from the other parent. In genetics, this represents a case of pseudodominance.
Discussion
Delta beta thalassemia is a rare form of thalassemia seen in different ethnic groups like Italians, Greeks, Turks and Asians [8]. Around nine mutations have been reported to be responsible for the condition of which Asian Indian Inversion deletion is found to be the most prevalent mutation in the Asian population. As the clinical manifestation of delta beta thalassemia resembles that of thalassemia major, thalassemia intermedia and hereditary persistence of fetal hemoglobin, the exact diagnosis of the condition requires proper genetic analysis of the patients as well as parents to identify the nature of mutation in cases where parents show high HbF levels in HPLC.
In the present study preliminary screening of the four patients for mutations in beta globin gene showed homozygosity for two different mutations (c.92+5G>C-three patients; c.315+1G>Aone patient) based on which they were diagnosed as thalassemia major. However, in-depth molecular analysis of the patients based on the HPLC findings of the parents revealed their genetic status as compound heterozygotes harbouring delta beta mutation being co-inherited with a beta gene mutation. In genetics, this is a rare phenomenon of psedodominance wherein the recessive allele though present in single dose on one chromosome is showing dominant pattern of inheritance due to presence of a deletion on other chromosome at that locus. For eg: In case of beta thalassemia two defective alleles (bb) are required to express the disease as it follows autosomal recessive mode of inheritance. However, in the present study the patients carried only one defective beta allele being inherited from one of the parents and Asian Indian Inversion deletion from the other parent on the homologous chromosome at the same locus.
So, these cases comply with the pattern of psedodominance in genetics.
Our cases showed high levels of fetal hemoglobin (78.8%, 79.5%, 76.7% and 96.7%) in contrast to the low HbF values reported by Pandey, et al, [9]. The HbF levels of our patients correlated with the one reported by Dehury, et al., but showed severe phenotype requiring transfusions [10]. Our results are consistent with Hariharan et al, who reported mean HbF levels of 90.0 ± 15.7 with lower hemoglobin levels, transfusion requirement and disease severity [11].
Another interesting feature observed in the present study was that the patient found to be compound heterozygous for Asian Indian Inversion deletion and c.315+1G>A had late onset of the disorder as compared to other three patients harbouring c.92+5G>C mutation in beta globin gene. The fetal hemoglobin in the three patients with c.92+5G>C (78.8%, 79.5%, 76.7%) was lower as compared to the levels in the patient with c.315+1G>A (96.7%) indicating that compound heterozygotes for Asian Indian Inversion deletion and beta mutation exhibit varying degrees of severity depending on the nature of beta mutation. The patients were also screened for different modifying factors like alpha deletion and XmnI polymorphism to rule out their influence on HbF levels. All the samples were found to be normal for alpha deletion and heterozygous for Xmn1 polymorphism. There was no effect of modifying factors on the phenotype of the patients. It is possible that high HbF levels detected in this patient might be ameliorating the severity of the disease as compared to other patients as hypothesized by many authors Hariharan et al., that elevated HbF ameliorates the clinical severity of β-thalassemia syndromes by restoring the globin chain imbalance. However, a larger sample size is required to support our results [11].
As individuals heterozygous for Hereditary Persistence of Fetal Hemoglobin (HPFH) also show high level of HbF levels (up to 30%), all the patients and parents were also analysed for Asian specific HPFH-6 and HPFH-3 deletion. None of the patients or parents showed the presence of HPFH deletion.
The above findings suggest that careful molecular analysis is required in cases where parents show high levels of fetal hemoglobin in HPLC. This would aid in providing accurate diagnosis, proper genetic counselling and treatment to the patients. Precise molecular characterisation of these conditions is of utmost importance in case of prenatal diagnosis and prevention programmes.
Conclusion
Careful examination of parents' Fetal Hemoglobin Levels (HbF) is mandatory to rule out delta beta thalassemia which may otherwise lead to wrong interpretation of the diagnosis of the child as thalassemia major. Accurate molecular diagnosis of the patient is therefore important to provide genetic counselling in case of prenatal diagnosis and also in prevention programs. Thus, the above cases highlight the importance of considering delta beta thalassemia in the presence of elevated HbF levels in the parents.
Acknowledgements
We are thankful to all the patients and their parents who participated in the study by giving their informed consent. Our sincere thanks to our President Mr Chandrakant Agarwal and all other board members for providing financial support for this study. We are also thankful to our staff without whose efforts this study wouldn’t have been possible.
References
- Carrocini GC, Ondei LS, Zamaro PJ, BoniniDomingos CR. Evaluation of HPFH and delta beta thalassemia mutations in a Brazilian group with high HbF levels. Genet Mol Res. 2011; 10:3213-9.
[Crossref], [Google Scholar]
- Pirastu M, Kan YW, Galanello R, Cao A. Multiple mutations produce delta beta 0 thalassemia in Sardinia. Science. 1984; 223:929-30.
[Crossref], [Google Scholar], [Indexing]
- Ramot B, Ben-Bassat I, Gafni D, Zaanoon R. A family with three beta-delta-thalassemia homozygotes. Blood. 1970; 35:158-65.
[Crossref], [Google Scholar], [Indexing]
- Supan F, Yutthana P, Satja S, Goonnapa F, Kanokwan S. Molecular and hematological characterization of hereditary persistence of fetal hemoglobin-6/Indian deletion –inversion Gγ(Aγδβ)0-thalassemia and Gγ(Aγδβ)0-thalassemia/HbE in Thai patients. American Journal of Hematology. 2002; 71: 109-13.
[Crossref], [Google Scholar], [Indexing]
- Vinodh Kumar B, Choccalingam C, Samuel P. Incidental identification of possible delta-beta thalassemia trait in a family: Arare cause of elevated Hb F. J Clin Diagnostic Res. 2016;10: BD01- BD02.
[Crossref], [Google Scholar], [Indexing]
- Sharma S, Sehgal S, Das R, Gulati S. Phenotypic heterogeneity of delta-beta thalassemia. Indian J Pathol Microbiol. 2019;62:185-186.
[Crossref], [Google Scholar], [Indexing]
- Craig JE, Barnetson RA, Prior J, Raven JL, Thein SL. Rapid detection of deletions causing delta beta thalassemia and hereditary persistence of fetal hemoglobin by enzymatic amplification. Blood. 1994;83:1673-82.
[Crossref], [Google Scholar], [Indexing]
- Huma M, Sidra A, Anila R, and Farheen K. Delta beta thalassemia: A rare haemoglobin variant. Blood Res. 2016;51:213-214.
[Crossref], [Google Scholar], [Indexing]
- Pandey S, Pandey S, Ranjan R, Mishra R, Sharma M, Saxena R. Phenotypic heterogeneity of Asian Indian inversion deletions Gc(Acdb) 0 breakpoint A and breakpoint B. Indian J Clin Biochem 2013; 28:98–101.
[Crossref], [Google Scholar], [Indexing]
- Dehury S, Purohit P, Meher S, Das K, Patel S. Compound heterozygous state of β-thalassemia with IVS1-5 (G→C) mutation and Indian deletion-inversion Gγ(Aγδβ)(0)-thalassemia in eastern India. Rev Bras Hematol Hemoter.2015;37:202-206.
[Crossref], [Google Scholar], [Indexing]
- Hariharan P, Kishnani P, Sawant P, Gorivale M, Mehta P, et al. Genotypic-phenotypic heterogeneity of δβ-thalassemia and Hereditary Persistence of Fetal Hemoglobin (HPFH) in India. Ann Hematol. 2020;99:1475-1483.
[Crossref], [Google Scholar], [Indexing]





 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.