Research Trends in Post‑Graduate Medical Students, Pune
- *Corresponding Author:
- Dr. Priya Kulkarni
5, Shanti Sadan, Tarte Colony, Erandawane, Pune ? 411 004, Maharashtra, India.
E-mail: dr_pdkulkarni@yahoo.co.in
Citation: Bhawalkar JS, Jadhav SL, Banerjee A, Kulkarni PY, Bayan P, Shachi A. Research trends in post-graduate medical students, Pune. Ann Med Health Sci Res 2014;4:355-60.
Abstract
Background: Scientific writings provide a link between production of knowledge and its use. They guide to plan for necessary improvements in treatment and prevention modalities. Inadequate and incomplete reporting of research studies weakens the medical literature. Aim: The aim of the study was bibliometric analysis of dissertations submitted by medical post‑graduate (PGs) students. Material and Methods: It was a cross‑sectional record based study carried out at one of the medical colleges at Pune. All the dissertations including observational studies submitted by PGs were analyzed using a pre‑tested, structured assessment tool with strengthening the reporting of observational studies in epidemiology (STROBE) as the reference from 1st March 2009 until 30th March 2011. Data was compiled in excel sheet and it was imported into. SPSS 15 software (Statistical package for the social sciences, manufactured by IBM, Chicago Illinois in November 2006) for further analysis. Results: A total of 220 dissertations were analyzed in the present study. Nearly 73.18% (161/220) of dissertations were from clinical subjects, 15% (33/220) were from para‑clinical and 11.8% (26/220) were from pre‑clinical subjects. The majority of dissertations reported findings incompletely and inadequately without uniformity. Conclusion: PGs should be more vigilant in reporting their research in dissertations to increase their usefulness. They should adopt tools like STROBE, etc., to report data to create more uniformity in reporting. Adoption of such guidelines will also reduce chances of oversights and mistakes in reporting and dissertation writing.
Keywords
Research trends, Scientific writings, Strengthening the reporting of observational studies in epidemiology
Introduction
Scientific writings in the particular field, based on international bibliographic data, are one of the most widely used methods to measure scientific achievement. Scientific writings like dissertations submitted by post‑graduate students (PGs) based on research studies serve an important role in the scientific process. They link between the production of knowledge and its use.[1,2]
The presentation of a dissertation has been one of the requirements for the PG medical degree in Pune and all over India. All PGs have to undertake some research work for their dissertation to be submitted before final examinations. A wide choice of subjects for research is permitted. Students may choose basic laboratory projects or may investigate clinical, epidemiologic or sociologic (including medicine and humanities) topics. Data must be subjected to statistical analysis to summarize results of research activity. Dissertations are evaluated according to study design, sample size, research instruments used, possible sources of bias, confounders etc., by the panel of external and internal examiners who grade it after viva of the student. However, yet there is no standard method for evaluation of dissertations is adopted by examiners. Different methods used by examiners may lead to evaluation error. It can be avoided if standard method like strengthening the reporting of observational studies in epidemiology (STROBE) is adopted to evaluate dissertations. It can help to improve the quality of reporting research through dissertations if PGs know such standardized tool of evaluation before‑hand.
PGs may publish their original research work and contribute to the medical literature. Hence, PGs should also take extreme care while designing a study as well as during preparation of dissertations based on their study results.
It is difficult to keep abreast with the large amount research added in biomedical literature as it is constantly growing day‑to‑day.[3] Reporting of objective information obtained from research studies should be complete, adequate and accurate. Otherwise, it is difficult to plan for necessary improvements in infrastructure related to treatment and prevention.
According to von Elm et al.,[4] much biomedical research is observational and reporting of such research is often inadequate. It hampers the assessment of its strengths and weaknesses of studies reported in the medical literature. It also adversely affects external validity of a research study. Readers need to know what was planned and what was not planned, what was done, what was found and what the results of the study mean. If some recommendations on the reporting of scientific studies will be followed quality of reporting of research studies can be improved with fulfillment of readers’ expectations.
STROBE Initiative developed recommendations on what should be included in an accurate and complete report of an observational study. STROBE stands for an international, collaborative initiative of epidemiologists, methodologists, statisticians, researchers and journal editors involved in the conduct and dissemination of observational studies, with the common aim of improving reporting of observational studies. It is well‑documented that the European Union and the United States are the leading powers in biomedical investigation and publications. The STROBE statement is being endorsed by a growing number of biomedical journals.[5]
STROBE Initiative developed recommendations on what should be included in an accurate and complete report of an observational study[4] [Table 1]. The STROBE recommendations are accompanied by an explanation and elaboration (E and E) document. It explains in detail the rationale of each item and provides examples of transparent reporting from published articles. Until date, the STROBE statement is endorsed by over 100 journals as well as by the International Committee of Medical Journal Editors.[5]
| Item no | Recommendation | |
|---|---|---|
| Title and abstract | 1 | Indicate the study’s design with a commonly used term in the title or the abstractProvide in the abstract an informative and balanced summary of what was done and what was found |
| Introduction | ||
| Background/rationale | 2 | Explain the scientific background and rationale for the investigation being reported |
| Objectives | 3 | State specific objectives, including any pre‑specified hypotheses |
| Methods | ||
| Study design | 4 | Present key elements of study design early in the paper |
| Setting | 5 | Describe the setting, locations, and relevant dates, including periods of recruitment, exposure, follow‑up,and data collection |
| Participants | 6 | Cohort study-Give the eligibility criteria, and the sources and methods of selection of participants.Describe methods of follow‑up. Case‑control study-Give the eligibility criteria, and the sources andmethods of case ascertainment and control selection. Give the rationale for the choice of cases andcontrols. Cross‑sectional study-Give the eligibility criteria, and the sources and methods of selection ofparticipants |
| Cohort study-For matched studies, give matching criteria and number of exposed and unexposed. | ||
| Case‑control study-For matched studies, give matching criteria and the number of controls per case | ||
| Variables | 7 | Clearly define all outcomes, exposures, predictors, potential confounders, and effect modifiers. Givediagnostic criteria, if applicable |
| Data sources/ | 8 | For each variable of interest, give sources of data and details of methods of assessment (measurement). |
| measurement | Describe comparability of assessment methods if there is more than one group | |
| Bias | 9 | Describe any efforts to address potential sources of bias |
| Study size | 10 | Explain how the study size was arrived at |
| Quantitative variables | 11 | Explain how quantitative variables were handled in the analyses. If applicable, describe which groupingswere chosen and why |
| Statistical methods | 12 | Describe all statistical methods, including those used to control for confoundingDescribe any methods used to examine subgroups and interactionsExplain how missing data were addressedCohort study-If applicable, explain how loss to follow‑up was addressed. Case‑control study-If applicable,explain how matching of cases and controls was addressed. Cross‑sectional study-If applicable, describeanalytical methods taking account of sampling strategyDescribe any sensitivity analyses |
| Results | ||
| Participants | 13 | Report numbers of individuals at each stage of study-e.g., numbers potentially eligible, examined foreligibility, confirmed eligible, included in the study, completing follow‑up, and analysedGive reasons for non‑participation at each stageConsider use of a flow diagram |
| Descriptive data | 14 | Give characteristics of study participants (e.g., demographic, clinical, social) and information onexposures and potential confoundersIndicate number of participants with missing data for each variable of interestCohort study-Summarise follow‑up time (e.g., average and total amount) |
| Outcome data | 15 | Cohort study-Report numbers of outcome events or summary measures over timeCase‑control study-Report numbers in each exposure category, or summary measures of exposureCross‑sectional study-Report numbers of outcome events or summary measures |
| confidence interval). Make clear which confounders were adjusted for and why they were included | ||
| Report category boundaries when continuous variables were categorized | ||
| If relevant, consider translating estimates of relative risk into absolute risk for a meaningful time period | ||
| Other analyses | 17 | Report other analyses done-e.g., analyses of subgroups and interactions, and sensitivity analyses |
| Discussion | ||
| Key results | 18 | Summarise key results with reference to study objectives |
| Limitations | 19 | Discuss limitations of the study, taking into account sources of potential bias or imprecision. Discuss bothdirection and magnitude of any potential bias |
| Interpretation | 20 | Give a cautious overall interpretation of results considering objectives, limitations, multiplicity of analyses,results from similar studies, and other relevant evidence |
| Generalisability | 21 | Discuss the generalisability (external validity) of the study results |
| Other information | ||
| Funding | 22 | Give the source of funding and the role of the funders for the present study and if applicable, for theoriginal study on which the present article is based |
Table 1: Strobe statement-checklist of items that should be included in reports of observational studies
Bruno et al. (2011) identified 643 citations of STROBE since its first publication in October 2007 until August 2010. Around 32% (234/643) citations concerned the E and E document of STROBE while 68% (439/643) citations concerned the checklist as per STROBE. The STROBE checklist published in the Lancet was the most cited, receiving 17% (110/643) citations. They also assessed 100 randomly selected articles. Out of them, 32% were observational studies and 19% were systematic reviews/ meta‑analyses. Comments, editorials and letters accounted for 15% of the articles, methodological articles for 8% and recommendations and narrative reviews for 26%. Of the 32 observational studies assessed, 81% (26/32) made appropriate use of STROBE and 10% (3/32) uses were considered as inappropriate. Of the 19 systematic reviews or meta‑analyses assessed, 53% (10/19) made an inappropriate use of STROBE.[2]
A systematic assessment of dissertations completed from a medical college in Pune, using an internationally recognized evaluation standard like STROBE is not available. Hence, we carried out the study with the primary objective of bibliometric analysis of dissertations submitted by PGs of one of the Medical Colleges in Pune using STROBE with the aim to assess whether such standardized and unique criteria is needed for evaluation of dissertations or not.
Subjects and Methods
It was a cross‑sectional record based study carried out at one of the Medical Colleges and Research Centers at Pune, Maharashtra, India. The college conducts PG degree and diploma courses in all pre‑clinical, para‑clinical and clinical subjects. PGs admitted to these courses are required to submit projects and/or dissertations before they appear for final university examination. We examined the dissertations reporting observational studies, written and submitted by PGs of selected Medical College in 2 years from 1st March 2009 until 30th March 2011. All the available dissertations submitted for PG medical degrees were selected. As STROBE is used to report observational studies we excluded dissertations reporting clinical trials.
Necessary permissions were obtained from the ethical committee of the institution and all the PGs who had submitted dissertations in the library of concerned Medical College. Dissertations were assessed using a pre‑designed and pre‑tested assessment tool with STROBE as the reference [Table 1]. Responses to each item of STROBE [Table 2] were binary in the form of Yes, No.
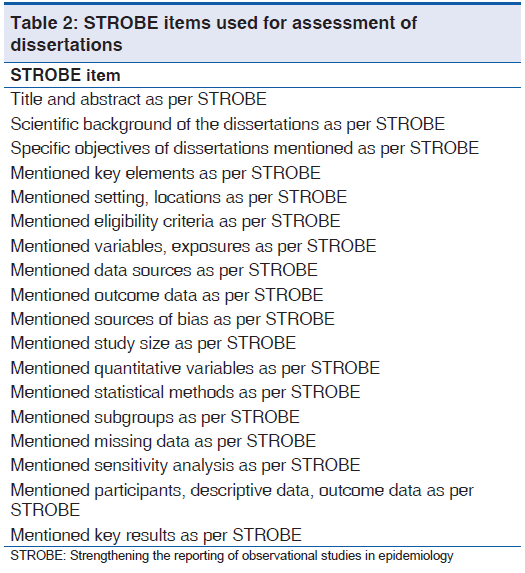
Table 2: STROBE items used for assessment of dissertations
Data were compiled in excel sheet and imported into statistical package for the social sciences. 15 (Chicago Illinois, USA) software for further analysis. Various characteristics of dissertations as per STROBE were enlisted as frequencies and percentage for assessment of dissertation from various specialties.
Each dissertation was scored as per STROBE checklist, 0 being the lowest score and 18 being highest. STROBE score 0‑12 was considered to be unsatisfactory while STROBE score from 13 to 18 was considered as satisfactory. Likelihood ratio test was used as test of significance to find if there was any association of satisfactory STROBE score with the clinical, pre‑clinical and para‑clinical specialties.
Results
Subject‑wise distribution of dissertations
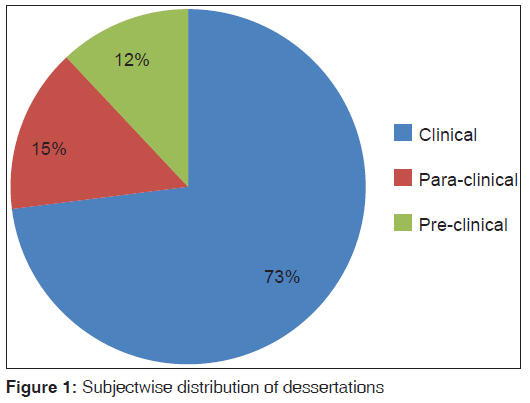
A total of 220 dissertations were analyzed in the present study. About 73.18% (161/220) dissertations were from clinical subjects, 15.00% (33/220) were from para‑clinical, 11.82% (26/220) were from pre‑clinical subjects [Figure 1]. Out of 220 dissertations analysed, 15% (33/220) were from genaral medicine and 12% (26/220) were from genaral surgery [Table 3].
| Subject | Number of dissertations submitted | Percentage |
|---|---|---|
| General medicine | 33 | 15 |
| General surgery | 26 | 12 |
| Obstetrics and gynaecology | 19 | 9 |
| Paediatrics | 18 | 8 |
| Orthopaedics | 16 | 7 |
| Anaesthesia | 13 | 6 |
| Radiology | 10 | 5 |
| Anatomy | 10 | 5 |
| Physiology | 11 | 5 |
| Pathology | 11 | 5 |
| Microbiology | 9 | 4 |
| Skin and VD | 9 | 4 |
| Ophthalmology | 8 | 4 |
| Biochemistry | 6 | 3 |
| Pharmacology | 7 | 3 |
| Community medicine | 5 | 2 |
| Psychiatry | 3 | 1 |
| Pulmonary medicine | 3 | 1 |
| Otolaryngology | 3 | 1 |
| Total | 220 | 100 |
Table 3: Subject‑wise distribution of the dissertations
Characteristics of dissertations as per STROBE
Majority, 74.5% (164/220) dissertations specified title, 95.90% (211/220) specified specific objectives satisfactorily as per STROBE. But, only 12.3% (27/220) summarized key results and present them by utilizing proper methods of data presentation. Eligibility criteria were not specified in 80% (176/220) dissertations [Table 4].
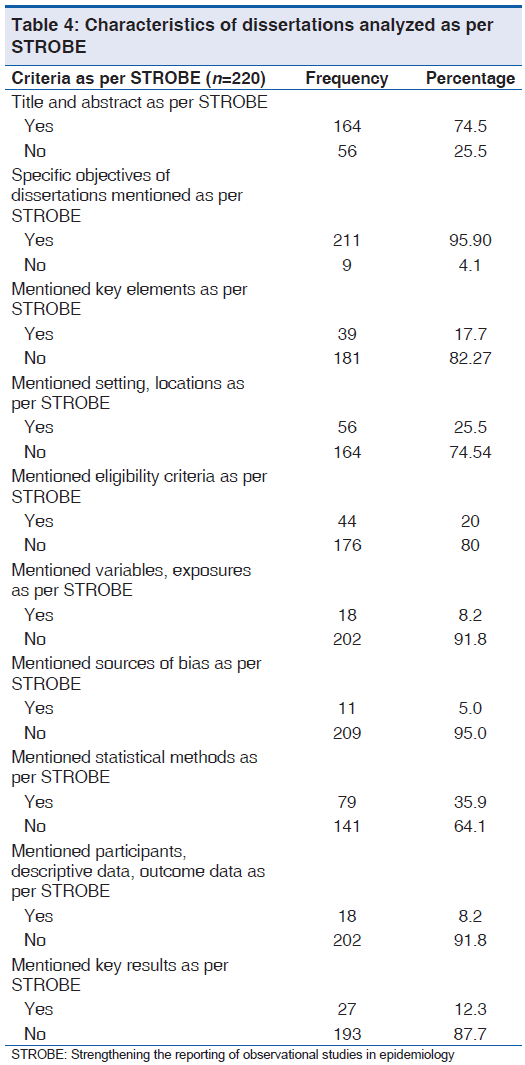
Table 4: Characteristics of dissertations analyzed as per STROBE
STROBE checklist was scored from 0 to 18, 0 being minimum score, 18 as maximum. STROBE score as 0‑12 was considered as unsatisfactory, 0 being most unsatisfactory while STROBE score as 13‑18 was considered as satisfactory, 18 being most satisfactory. Mean STROBE score came out to be 11 (+2.63). Overall, 27.37% (60/220) dissertations had satisfactory STROBE score while 72.63% (160/220) had unsatisfactory STROBE score. Satisfactory STROBE score was not associated with the clinical, pre‑clinical or para‑clinical subjects (P = 0.093).
Discussion
Although STROBE is used primarily for assessment of published articles, we found it useful for reviewing unpublished work, like dissertations submitted by PGss. This is the first such study of its kind.
Bibliometric analysis of dissertations by PGs of selected Medical College and Research Center by using STROBE in the present study found that there was no uniformity in reporting the research studies in dissertations. This irregularity makes it very difficult to review and study these dissertations.
We observed dissertations were very good in indicating title, specific objectives and study design. However, majority of them were unable to summarize key results and present them by utilizing proper methods of data presentation [Table 4].
A considerable proportion of dissertations were less vigilant in reporting the findings. Bogardus et al., had argued that without sufficient clarity of reporting, the benefits of research might be achieved more slowly.[6] If the students had used STROBE or any other tool as a guideline such an over sight could have been avoided. This observation supports the inference drawn by Rennie who stated that there is a need for guidance in reporting observational studies.[7,8]
In this study, out of 220 dissertations analyzed only 20.0% (44/220) specified the eligibility criteria, sources and methods of case ascertainment and control selection and 80% (176/220) did not. Tooth et al. stated, in a survey of longitudinal studies in stroke research, 35% articles (17/49) did not specify the eligibility criteria.[9]
A study on reporting of epidemiologic studies found that the participant selection process – for example, information on exclusions and refusals – often lacked details. He also found that the selection of controls and adjustment for potential confounders needs greater clarity, consistency and explanation.[10] We also observed these insufficiencies in the majority of dissertations.
Only 5.0% (11/220) dissertations described any efforts to address potential sources of bias and only 1.4% (3/120) explained how missing data were addressed.
World‑wide, there is a need of reporting medical research from India and other developing countries. Indian doctors lag behind inpublishing their research work. To boost publications, the Medical Council of India has stipulated minimum number of publications by faculty and postgraduate students. Eventually, there is a growing concern of scientific publications in India and majority of PGs may consider publishing their research work for dissertation in future. Adaptation of standard guidelines like STROBE will improve the quality of publication besides increasing the quantity.
According to Chaudhuri[11] and Nayak,[12] national journals should be encouraged and supported for publishing the work of Indian authors. Publications in high impact factor journals are necessary for the advancement of Indian medical and scientific profession. Publications in high impact factor journals truly represent the best international content. Their editors actively seek the best articles after evaluation. Publications resulting from international collaborations increased from 3% in 2001‑8% in 2006 in India.
Study by Kumaragurupari examined scientific publications generated by ophthalmologists, optometrists and researchers working in vision science in India through a systematic search of Medline using the PubMed interface. A total of 2163 related articles were published from 2001 to 2006. During the 6‑year period studied, the annual output of research articles has nearly doubled, from 284 in 2001‑460 in 2006. Two‑thirds of these were published in international journals; nearly 41% in vision‑related journals with high impact factors and 3% in impact factor journals, which were not vision‑related. Around 50% of the publications came from nine major eye hospitals. Clinical science articles were most frequently published whereas basic science the least.[1] Hence, PGs specially from clinical specialties should follow some standard international guidelines to report and publish their research.
PGs should also have access to international journals to get the content of international publications. Concerned institutions should subscribe them regularly. According to a survey by Murthy et al.,[13] subscriptions to international journals were inadequate in most of the institutions in India, their research in 2002‑03 showed that only 53 of 128 surveyed institutions in India subscribed to more than two journals.
International collaborative research supports many scientists and clinicians from India and has a growing record of biomedical accomplishment.[14] According to Kupfer et al. (1995)[15] many Indian researchers now have experience in collaborating with researchers in the US and other western countries. They bring their own expertise, for instance, many have extensive experience in rural outreach, an important asset considering the need for comprehensive epidemiologic data and populations with both common and rare genotypes. Western scientists stand to gain enormously from access to patients and expertise of their Indian collaborators.
This analysis will be helpful to find out the obstacles of research productivity, which would help to develop research quality and capacity. The shortfalls of observational epidemiology in terms of the generation of contradictory and spurious findings have been highlighted by many commentators. A general practitioner, James Le Fanu, stated that “the simple expedient of closing down most University departments of Epidemiology could both extinguish this endlessly fertile source of anxiety‑mongering while simultaneously releasing funds for serious research” The abandonment of observational epidemiology might be premature without first trying some remedial steps. One important step would be to improve the reporting of observational epidemiological studies.[16]
In the present study, we could not get a representative sample of dissertations from all medical colleges in Pune. Results may be applicable to the medical college from which dissertations are analyzed. However, it suggests that there is a need of adoption of some research tool by PGs, ultimately it will help to improve medical literature. Other limitation of the study is that it assessed dissertations reporting only observational studies. Dissertations reporting experimental studies needs separate tools like CONSORT.
We would like to recommend that PGs should be more vigilant in reporting of their research, there should be more transparency in the dissertations. All projects should state generalizability of their study and its findings. PGs should study how assessment of dissertations will be made after completion of dissertations so that they will not miss any important point to report. They should be made aware of assessment tools such as STROBE, CONSORT (Consolidated Standards of Reporting Trials) and PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‑Analyses) that may be adopted by universities to evaluate dissertations. It will help to improve study design of their research. For this purpose, help from clinical research faculty and statisticians should be taken beforehand while designing research study.
Adoption of such tools by PGs and their guides will help to create more uniformity in reporting. It might help temper the over‑enthusiastic reporting of new findings in the scientific community and popular media and improve the methodology of studies in the long‑term.[3]
References
- Kumaragurupari R, Sieving PC, Lalitha P. A bibliometric study of publications by Indian ophthalmologists and vision researchers, 2001‑06. Indian J Ophthalmol 2010;58:275‑80. Available from: http://www.ijo.in. [Last accessed on 2011 Apr 28].
- Bruno R, Myriam Cevallos, Douglas G Altman, Anne WS. Uses and misuses of the STROBE statement: Bibliographic study. Bmj open 2011;1:e000048. Available from: http:// www.bmjopen.bmj.com. [Last accessed on 2011 Apr 27].
- Thrift AG. Systematic review of observational studies. Neuroepidemiology 2010;34:262‑3.
- von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. BMJ 2007;335:806‑8.
- STROBE Statement. Strengthening the reporting of observational studies in epidemiology. Available from: http:// www.STROBE‑statement.org. [Accessed on 2010 Dec 15].
- Bogardus ST Jr, Concato J, Feinstein AR. Clinical epidemiological quality in molecular genetic research: The need for methodological standards . JAMA 1999;281:1919‑26.
- Guidelines for documentation of epidemiologic studies. Epidemiology Work Group of the Interagency Regulatory Liaison Group. Am J Epidemiol 1981;114:609‑13.
- Rennie D. CONSORT revised – Improving the reporting of randomized trials. JAMA 2001;285:2006‑7.
- Tooth L, Ware R, Bain C, Purdie DM, Dobson A. Quality of reporting of observational longitudinal research. Am J Epidemiol 2005;161:280‑8.
- da Costa BR, Cevallos M, Altman DG, Rutjes AW, Egger M. Uses and misuses of the STROBE statement: Bibliographic study. BMJ Open 2011;1:e000048.
- Chaudhuri BR. National policy for science journals. J Indian Med Assoc 1977;68:16‑8.
- Nayak BK. Why should you publish in the Indian Journal of Ophthalmology? Indian J Ophthalmol 2008;56:451‑2.
- Murthy GV, Gupta SK, Bachani D, Sanga L, John N, Tewari HK. Status of speciality training in ophthalmology in India. Indian J Ophthalmol 2005;53:135‑42.
- India scientific research continues to grow: Thomson Reuters analyzes India’s growing share of world’s scientific papers [Internet]. Philadelphia: Vriti group online posts, Nov 2011. Available from: http://www.goiit.com/posts.
- Kupfer C. Collaboration between the National Eye Institute and Indian scientists in vision research. Indian J Ophthalmol 1995;43:45‑6.
- Thrift AG. Systematic review of observational studies. Neuroepidemiology 2010;34:262‑3.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.