Retrospective Cohort Study on the Effectiveness of Remdesivir in the Treatment of COVID 19 Positive Patients Admitted at Government Medical College and Hospital, Akola, Maharashtra
2 Department of Radiodiagnosis, Government Medical College, Akola, Maharashtra, India
3 Department of Pharmacology, Government Medical College, Akola, Maharashtra, India
4 Department of Community Medicine, Government Medical College, Akola, Maharashtra, India
Received: 01-Nov-2021 Accepted Date: Nov 09, 2021 ; Published: 21-Nov-2021
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Keywords
Cohort; Clinical improvement; Follow up; Outcome; Ordinal scale; Remdesivir
Introduction
Coronaviruses are large group of viruses that cause illness in humans and animals. Novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was first identified during mid-December 2019 in a seafood market of Wuhan city in Hubei Province of China [1].
Since the first cases were reported infection with the severe acute respiratory coronavirus 2 (SARS-CoV-2) has become a worldwide pandemic. [1,2] The huge burden on health system due to illness caused by SARSCoV- 2 leads to difficult in coping the pandemic globally. [3,4] The symptoms of SARS-CoV-2 infection vary widely, from asymptomatic disease to pneumonia and life-threatening complications, including acute respiratory distress syndrome, multisystem organ failure, and ultimately, death.
[5-7] older patients and those with preexisting respiratory or cardiovascular conditions found to be at the greatest risk for severe complications. [6, 7] At present proven effective therapy for SARS-CoV-2 is not documented. In the absence of an effective therapy, current management consists of supportive care, including invasive and noninvasive oxygen support and treatment with antibiotics. [8,9] In addition, many patients have received off-label or compassionate- use therapies, including antiretrovirals, antiparasitic agents, antiinflammatory a compounds, and convalescent plasma.
The anti-inflammatory drugs like tocilumab, itolizumab and antiviral falvipiravir, hydroxychloroquine are used [10-13]despite the question mark about their efficacy in covid 19 treatment. Use of these therapies is based on limited available evidence. Remdesivir has broad-spectrum activity against members of several virus families, including filoviruses (e.g., Ebola) and coronaviruses (e.g., SARS-CoV and Middle East respiratory syndrome coronavirus [MERS-CoV]). [14] Remdesivir is a prodrug of a nucleotide analogue that is intracellularly metabolized to an analogue of adenosine triphosphate that inhibits viral RNA polymerases. WHO has issued a conditional recommendation against the use of Remdesivir in hospitalized patients?[15-17].
The use of Remdesivir in treatment of covid 19 infection was failed to reach consensus in various countries and organizations.
The ‘Adaptive Covid –19 Treatment Trial’ found that Remedesivir led to a shorter median time from randomization to recovery (10 days, vs. 15 days with placebo) and reduced the time to hospital Recovery (12 days vs. 17 days) but did not show a mortality benefit. [18].
Whereas The ‘Solidarity Trial’ conducted by WHO in 30 countries from March 2020 at 405 hospitals showed that Remdesivir had little or no effect on hospitalized patients with COVID-19, as indicated by overall mortality, initiation of ventilation, and duration of hospital stay. [19] Joint Monitoring Group under Chairmanship of DGHS in India released advisory based on findings of Adoptive trial and Solidarity trial for Physicians/ Doctors to exercise extreme caution in using this reserve/ experimental/ emergency use authorisation drug Remdesivir to stop it’s misuse as this is only an experimental drug with potential to harm, has relatively high cost and has limited availability. [18, 19].
According to currently available evidence, we still do not know the benefit or harm of Remdesivir treatment in severe COVID-19 patients. However, further studies of Remdesivir in patients with COVID-19 might help us to better understand its potential mechanism and clinical efficacy. The present study was planned with an objective to know clinical improvement and outcome in a cohort of covid 19 positive patients who were treated with Remdesivir.
Materials and Methods
The present retrospective cohort study conducted among Covid 19 positive patients admitted at Government Medical College and hospital, Akola over a period of six months from November 2020 to April 2021 who received injection Remdesivir as treatment modality. The patients who had SARS-CoV-2 infection confirmed by Reverse-transcriptase– polymerase-chain-reaction assay (RtPCR) above age of 18 years and either an oxygen saturation of 94% or less while the patient was breathing ambient air and/ or showing radiological evidence of pulmonary Infiltrates or a need for oxygen support were included in the study . Liver function tests were obtained in all patients before administration of Remdesivir and during treatment as clinically indicated. Remdesivir was discontinued if alanine transaminase (ALT) levels increase to >10 times the upper limit of normal and signs or symptoms of liver inflammation were observed. The treatment protocol followed in the hospital under study was 5 days course of Intravenous Injection of Remdesivir which includes dose of 200 mg on Day 1 followed by 100 mg for 4 days. Concomitant Medications given were corticosteroids, antibiotics, vitamin supplementation and anticoagulants as per need.
A total of 953 out of 3974 covid 19 positive admitted patients received Injection Remedesivir as treatment modality at Government Medical College, Akola from November 2020 to April 2021. Out of 953 patients, who received Remdesivir injection, 194 patients were excluded from study due to referral to other institute for treatment and no further information or incomplete information available of them. Finally by purposive sampling 759 patients who received Injection Remdesivir treatment at Government Medical College, Akola was included in present study.
Data Collection Instruments
Predesigned and pretested proforma was used for data collection. Google form on Google webpage was designed from validated proforma for ease of data collection and data entry. The data abstracted from the patient's medical case records available at Medical Record section of the institute. The information on socio-demographic characteristics like age, sex, religion, place of residence along with duration of symptoms before initiation of treatment with Remdesivir was obtained. The follow up information on key clinical events, respiratory rate and other vital parameters, type of oxygen supplementation including changes in oxygen-support requirements, concomitant medications, adverse events, and laboratory values, including serum creatinine, ALT, and AST, were recorded on day 1, day 3, day 8 and day 10. The follow- up was continued for at least 30 days after the beginning of treatment with Remdesivir or until Recovery or death whichever was earlier to know end point of the clinical improvement.
The primary outcome measure was observed on 10th day after Remdesivir treatment on modified ordinal scale (as per WHO R and D print). [15, 20] and again measured on day 30 in person for hospitalized patients or by Telephonic call for those who had been recovered. Criteria for clinical improvement were defined at 30 days by live Recovery from the hospital, a decrease of at least 2 points from baseline on a modified ordinal scale. The six-point scale included the following categories: 1. not hospitalized; 2. hospitalized, not requiring supplemental oxygen; 3. hospitalized, requiring supplemental oxygen; 4. hospitalized, requiring nasal high- flow oxygen therapy, noninvasive mechanical ventilation, or both; 5. hospitalized, requiring invasive mechanical ventilation, Extracorporeal membrane oxygenation (ECMO), or both; and 6. Death. Ethical approval was obtained from Institutional ethical committee for conducting the study.
Statistical Analysis
Chi square test of association was used to compare the proportion distribution of categorical variables. p value of less than 0.05 was used for statistical level of significance. Kaplan Meier survival analysis was used for assessing cumulative incidence of Clinical improvement (recovery rate) i.e. Hazard ratio with their confidence interval at the end point of follow up. Cox proportional hazards regression analysis was done to find out independent factors associated with clinical improvement.
Results
Total 759 patients who received Injection Remdesivir treatment at Government Medical College, Akola were included in present study. Table no I shows socio demographic profile of 759 study participants. Median age of patients was 56 years with range of 45 to 65 years. Maximum i.e. 367 (48.4%)patients were in the age group of 46-65 yrs followed by 197 (26%)patients in the age group of 26-45 years. A total 499(65.7%) were male patients in the study and 260 were females (34.3%). Majority of the participants were Hindu by religion i.e 635 (83.7) as compared to Muslim and Buddhist. Out of total patients 398(52.4%) were from rural area and 361(47.6%) were from Urban area.
| Table No I: Sociodemographic profile of study participants. | ||
|---|---|---|
| Variable | Number | Percentages |
| Age(yrs) ⋝25 26-45 46-65 >65 Median Age(yrs) (IQR) |
14 197 367 181 56(45-65) |
1.8 26.0 48.4 23.8 |
| Sex Male Female |
499 260 |
65.7 34.3 |
| Religion Hindu Buddhist Muslim Other |
635 91 28 05 |
83.7 12.0 3.70 0.70 |
| Residence Urban Rural |
361 398 |
47.6 52.4 |
| Table No II: Baseline Clinical characteristics of patients on Remdesivir treatment. | ||
|---|---|---|
| Variable | Number | Percentages |
| Co morbidity Hypertension Diabetes CKD Asthma Malignancy Other |
133 122 17 13 04 38 |
55.4 50.8 7.08 5.4 1.6 15.8 |
| Baseline clinical status Not required Oxygen Supplementation On Low flow Oxygen Supplementation On High Flow oxygen/Noninvasive Oxygen supplementation On Invasive ventilation |
135 300 302 22 |
17.8 39.5 39.8 2.90 |
| Concomitant medications Corticosteroids Antivirals( Favipiravir) Azithromycin Piperacillin and tazobactum Meropenem Anticoagulants |
650 45 314 173 57 239 |
85.6 5.92 41.4 22.8 7.50 31.5 |
| Median Duration of hospitalization before start of Remdesivir(IQR) | 1.0(1-2) | |
| Median Duration of symptoms before starting Remdesivir therapy(IQR) | 4.0(3-5) | |
| Median duration of hospital stay (IQR) | 8.0(5-10) | |
| Baseline Laboratory parameters –Median(IQR) Median SGOT(IU/L) Median SGPT(IU/L) Median Creatinine( mg%) |
33(22-54) 29(22-45) 1.0(1-1.1) |
|
As shown in Table no. (II). It was observed that out of 759 patients who were hospitalized for treatment majority of them i.e. 302 required noninvasive oxygen supplementation or High flow oxygen (39.8%) and 300 (39.5%)patients required low flow oxygen supplementation at the baseline scale before initiation of treatment with Remdesivir. A total of 135 patients did not required any oxygen supplementation and were on ambient air, whereas 22 (2.9%) patients were on invasive ventilation before the initiation of Remdesivir treatment.
The median duration of hospitalization before the initiation of Remdesivir treatment was 01 days (IQR: 1day -2 days) .This indicated patients received treatment within few days after hospitalization. Out of total 240 patients those were having existing comorbid conditions; hypertension (55.4%) was the most prevalent co morbidity followed by diabetes (50.8%).The proportion of chronic kidney disease was found among 7.08% of patients whereas asthma and malignancy was present in 5.4 % and 1.6% of patients respectively. The patients having other c omorbid conditions like hypothyroidism, liver disease Ischemic heart disease were 15.8%.
It was observed that 85.6% received corticosteroids as concomitant medications .Majority of the patients received antibiotics as Azithromycin (41.4%) and combination of piperacillin and tazobactum (22.8%) whereas anticoagulants were received by 31.8% of the patients.
| Table No III: Association of socio demographic factors with treatment outcome of | ||||
|---|---|---|---|---|
| study participants | ||||
| Variable | Recoveryd(n=497) | Death(n=262) | Total(n=759) | p value |
| Age(yrs) | ||||
| <25 | 12(85.7) | 2(14.3) | 14 | |
| 26-45 | 160(81.2) | 37(18.8) | 197 | 0.001* |
| 46-65 | 243(66.2) | 124(33.8) | 367 | |
| >65 | 82(45.3) | 99(54.7) | 181 | |
| Sex | 499 | 0.968 | ||
| Male | 327(65.5) | 172(34.5) | 260 | |
| Female | 170(65.4) | 90(34.6) | ||
| Religion | 635 | 0.001* | ||
| Hindu | 436(68.7) | 199(31.3) | 91 | |
| Buddhist | 46(50.5) | 45(49.5) | 28 | |
| Muslim | 12(42.9) | 16(57.1) | 5 | |
| Other | 03(60.00) | 2(40.0) | ||
| Residence | 239(59.6) | 162(40.4) | 401 | 0.001* |
| Rural | 258(73.5) | 93(26.5) | 351 | |
| Urban | ||||
Table no(III )It was found that proportion of recovery was significantly higher (85.7%)below 25 years of age as compared to proportion of recovery (45.3%) in the age group above 65 yrs. The proportions of recovery were almost equal in both sexes. The association between sex and outcome with treatment of Remdesivir was not statistically significant (p>0.05). Out of total 635 Hindu patients 68.7% were recovered from the hospital and 31.3% died after treatment with remedesivir. While considering religion, the proportion of death was higher in Muslim (57.1%) and Buddhist religion (49.5%) as compared to Hindu religion. The difference between religion and treatment outcome was statistically significant (p<0.05). Out of total 351 patients those residing in urban area, majority of them (73.5 % ) were recovered and 26.5% were died after treatment whereas out of total 401 patients ,59.6 % were recovered and 40.4% died after treatment .The Observed difference was found statistically significant (p<0.05).
| Table No IV: Association of clinical characteristics with treatment outcome. | ||||
|---|---|---|---|---|
| Variable | Recoveryd (n=497) |
Death (n=262) |
Total (n=759) |
p value |
| Comorbidity Yes No |
77(32.1) 420(80.9) |
163(67.9) 99(19.1) |
240 519 |
0.001* |
| Duration of symptoms before treatment initiation (days) <5 >5 |
409(67.6) 88(57.1) |
196(32.4) 66(42.9) |
605 154 |
0.018* |
Table IV shows association of clinical status of patients with outcome of treatment. It was found that out of total 759 patients 240 were having existing comorbidity. Out of 240 those having co morbidities a total of 163(67.9%) were died even after initiation treatment with Remdesivir and only 77(32.1%) were recovered. The observed difference was statistically significant (p<0.05). Those patients having duration of onset of symptoms less than 5 days and started Remdesivir treatment proportion of recovery was higher i.e. 409 (67.6%) than death rate whereas those having history of onset of symptoms more than 5 days the proportion of recovery was 57.1 % as compared to 42.9% were died. The observed difference was statistically significant (p>0.05).
| Table No. V: Clinical status on ordinal scale before and after treatment with Remdesivir treatment on 10th day. | |||||||
|---|---|---|---|---|---|---|---|
| Ordinal Scale After treatment with Remdesivir day 08 |
Ordinal Scale | Baseline Ordinal scale before start of Remedesivir | Total | ||||
| Invasive (n=22) | Noninvasive (n=302) | Low flow oxygen (n=300) |
Ambient Air (n=135) | ||||
| 5 | 4 | 3 | 2 | ||||
| Death | 6 | 22 | 190 | 7 | 1 | 220 | |
| Invasive | 5 | 0 | 07 | 0 | 0 | 03 | |
| Noninvasive | 4 | 0 | 30 | 6 | 3 | 43 | |
| Low Flow Oxygen | 3 | 0 | 14 | 73 | 36 | 123 | |
| Ambient Air | 2 | 0 | 30 | 116 | 52 | 198 | |
| Recoveryd | 1 | 0 | 31 | 98 | 43 | 172 | |
| CLINICAL IMPROVEMENT ON DAY 10 | 0 | 75 | 214 | 95 | 384 | ||
| CLINICAL IMPROVEMENT ON DAY 29 | 0 | 78 | 288 | 131 | 497 | ||
Table no. V revealed that out 135 of those who were on ambient air at the baseline 31.8% were recovered after treatment, 38.5% remained on ambient air, 26.6% were shifted to low flow oxygen supplementation.
However only three patients worsened to low flow oxygen supplementation and death was found in only one patient of the patients. The clinical improvement was seen in 70.3% of the patients at the end of 10th days of follow up and 97.0% at the end point of Day 30.
Out 300 of those who were on low flow oxygen supplementation at the baseline 32.6% were recovered after treatment, 38.6% were shifted to ambient air. The proportion of death was 2.3% in them.
The clinical improvement was seen in 214 patients (71.3%) at the end of 10th days of follow up and clinical improvement was 96.0% at the end point of Day 30.
Out 302 of those who were on noninvasive ventilation at the baseline 10.26% were recovered after treatment, 9.93% were shifted to ambient air, 4.63% were shifted to low flow oxygen supplementation. The death was found in 190(62.9%) of the patients.
The clinical improvement was seen in 75 patients (24.8%) at the end of 10th days of follow up and 25.8% at the end point of Day 30.
A total of 22 patients were on invasive ventilation at the baseline. The death was found in all of them (100%). Thus no clinical improvement was observed in invasive ventilation.
Out of 759, a total of 384 ( 50.4% ) patients that showed clinical improvement on Day 10 of follow up and total of 497(65.4%) that showed clinical improvement on Day 30 i.e. at the end point of follow up and recovered and 262(34.5%) patients died even after treatment with Remdesivir.
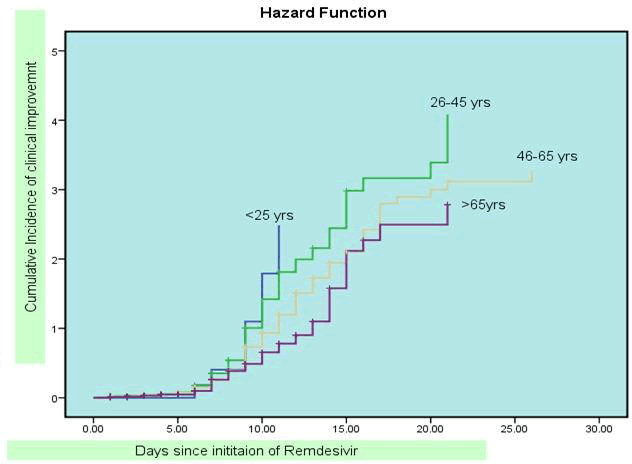
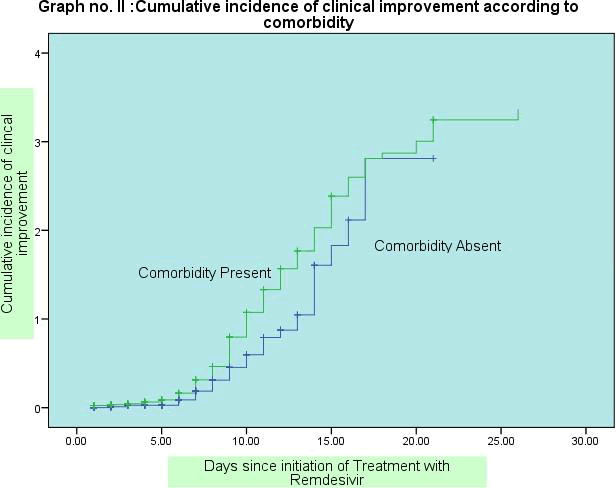
Graph I, II and III shows survival curves for clinical improvement according to age, comorbidity and baseline score: By applying cox proportion regression analysis, the clinical improvement was significantly higher in age group below 45 years as compared to the age group above 45 years and above ( hazard ratio 1.38, CI 1.07-1.77 ). The clinical improvement was higher in females as compared to males (Hazard ratio 0.89, CI 0.70-1.13) but on regression analysis not found significant association. Median t ime for clinical improvement was 9 days among Hindu patients. The clinical improvement was not significantly higher in patients those were Hindu By religion as compared to other religion (Hazard ratio 1.27, CI0.90 -1.79) on regression analysis.
The Median time for clinical improvement was 9 days among those from urban area and 10 days for rural population. On Regression analysis, the clinical improvement was not significantly higher in patients those were residing at Urban area as compared to those from Rural Area and (Hazard ratio- 1.18;CI0.94-1.48) the clinical improvement was significantly poor among those with comorbid condition as compared to those who were without preexisting comorbid condition. (Hazard ratio- 0.72, CI 0.53 -0.99) on regression analysis. The Median time of recovery was 11 days in co morbid patients and 9 days among those without any comorbidity.
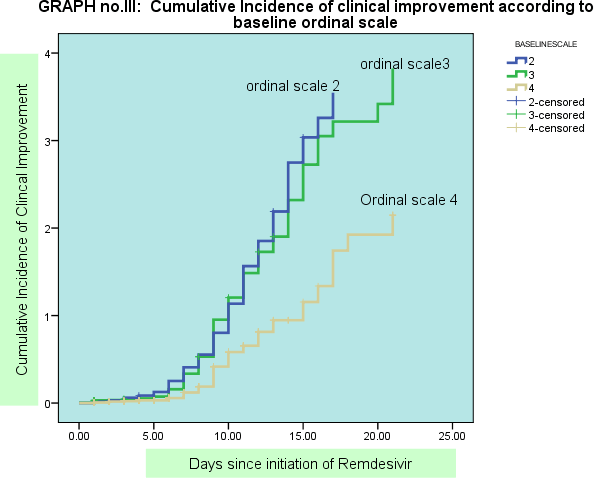
The clinical improvement was higher among those patients having duration of symptoms less than 5 days before initiation of treatment with Remdesivir. (Hazard ratio 0.93 CI; 0.68-1.2 ). The clinical improvement was significantly higher among the patients in those having baseline ordinal scale 2 i.e those who were on low oxygen saturation on ambient air and those on low flow oxygen supplementation at baseline as compared to those on noninvasive and invasive ventilation at baseline before initiation of treatment with Remdesivir (Hazard ratio-2.03, CI 1.01-2.74) on regression analysis.
Out of total 759 patients, 71(9.35%) patients developed adver se effects that lead to discontinuation of treatment with Remdesivir. Out of 71 patients who developed adverse effects 22 patients died and 49 patients were recovered. When observed the adverse effects of Remdesivir, it was found that a total 28(3.68%) patients’ hepatic enzymes SGOT and SGPT levels were raised after treatment with Remdesivir. Serum Creatnine level was raised in 22(2.89%) patients. It was observed that total 16% of the patients developed hypoxia and hypotension on next day after treatment with Remdesivir.
Discussion
Present retrospective cohort study was conducted to find out outcome of treatment with Remdesivir. In the present study maximum number of patients was in the age group of 46-65 yrs with median age of 54 yrs. The Recovery rate was higher below the age group of 45 years whereas death rate was higher above age of 65 yrs. These findings were similar to study by Beigel et al.19The patients with advanced age group are at greater risk of progression to severe disease even after treatment due to existing cormorbid conditions and age related factors.
The death rate was higher in patients from rural areas than urban areas. In urban areas tertiary level of health care facilities are easily accessible and timely available as compared to rural areas. Hence timely visit to health care centre leads to early treatment initiation and better outcome in urban population.
The risk of death was higher in those patients having existing comorbid conditions. The preexisting comorbid disease further detoriates the clinical condition in case of Covid 19 infection even after Remdesivir treatment. In the present study majority of patients were having hypertension and diabetes as preexisting cormorbid condition. The similar findings were noted in other study. [19]
The Recovery rate was higher in the patients having duration of symptoms less than 5 days before initiation of Remdesivir treatment as compared to those having duration of symptoms more than 5 days. Mahajan L et al [21] also found that recovery rates were higher among patients who had symptoms for less than 5 days before receiving treatment. Thus hospitalization and initialization of treatment with Remdesivir at the earliest would lead to favorable outcome.
We found the recovery rate was 65.4 % and mortality was 34.5% of the patients who received Remdesivir. It was found that clinical improvement was higher i.e. 97.0% and 96% among those who were on ambient air and those who were on low flow oxygen supplementation at the baseline respectively as compared to those requiring noninvasive and invasive ventilation at the baseline .Thus Remdesvir found to be effective when administrated at earlier stage of illness when patients on low oxygen saturation at ambient air or at low flow oxygen supplementation. In a open-label, randomized study of Remdesivir in hospitalized patients with moderate-severity Covid-19 , patients who received Remdesivir for 5 days had higher odds of clinical improvement than those receiving standard care (odds ratio, 1.65; 95% CI, 1.09 to 2.48; P = 0.02). [22]
Need for new mechanical ventilation or noninvasive ventilation was lower among those who had not receiving these at the baseline in present study .Similarly Beigel et al. [19] found the incidence of new mechanical ventilation or ECMO use among patients who were not receiving these interventions at enrollment was lower in the Remdesivir group than in the placebo group (13% [95% CI, 10 to 17] vs. 23% [95% CI, 19 to 27]) In ACTT-1, patients breathing ambient air or nasal cannula oxygen benefitted from treatment with Remdesivir, whereas patients receiving higher levels of respiratory support, such as mechanical ventilation, did not benefit.19Grein J et al found that improvement in oxygen- support status was observed in 68% of patients. [20] These findings are consistent with present study Mahajan L et al. [21] in their prospective randomized trial found that Remdesivir therapy for five days did not produce improvement in clinical outcomes in moderate to severe COVID‑19 cases. These results are contradictory to our findings in study. There is need randomized control trial with adequate sample size to clear the picture in Indian Context.
In present study time to clinical improvement was 8 days (IQR 5-10) .Garibaldi et al. [23] revealed that patients who received Remdesivir achieved clinical improvement before 28 days, with a median time to clinical improvement of 5.0 days (IQR, 4.0-8.0 days). In a study at China by Wang y et al. [24] early in the pandemic and showed a shorter time to improvement (a two-point improvement) with Remdesivir: 21.0 days (95% CI, 13.0 to 28.0) in the Remdesivir group and 23.0 days (95% CI, 15.0 to 28.0) in the placebo group (hazard ratio for clinical improvement, 1.23; 95% CI, 0.87 to 1.75).
Limitation
Ours is retrospective observation study based on data abstraction from hospital case records. Most of the information was missing or incomplete as heavy work load during the pandemic situation. Hence complete information about adverse effects could not be retrieved. Also the observed adverse effects were due to Remdesivir treatment or due to disease condition progression itself couldn’t be differentiated from case records as laboratory parameters were increased in both conditions. The patients who were referred to other centre the treatment outcome for those were not studied. The covid 19 positive patients in the present study received concomitant medications as per the need Hence recovery after treatment was only due to Remdesivir drug or concomitant medications had additional benefit of recovery could not be differentiated from the present study.
Other factors may had contributed to differences in treatment outcomes including the variat ions in ventilatory practices and trained manpower availability, adequate infrastructure to cope up with existing pandemic .There is need of multicentric prospective Randomized controlled trial in Indian context to support the treatment benefits of Remdesivir .
Conclusion
The clinical improvement was observed in majority of the covid 19 positive patients who received t reatment with Remdesivir. Remdesivir treatment associated with higher chances of recovery as well as reducing duration of hospital stay. However the treatment outcome was poor among the patients with advanced age group and preexisting morbidity. There is need to protect the elderly people from infection with Covid 19 by boosting their immunity through proper nutrition during the pandemic along with use of appropriate preventive strategy. The patients with low oxygen saturation at ambient air and required low flow oxygen supplementation at the baseline were having early clinical improvement and higher Recovery rate. Thus clinical improvement was observed in those patients who approached the health centre at early stage of disease before worsening of the clinical condition. The patients with mild symptoms who are home quarantined should be provided a well-equipped kit containing pulse oximeter, basic medicines and early identification of warning signs to seek the health centre immediately. This will help for init iation of treatment at earlier stage of disease.
References
- Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed 2020; 91:157-160.
- Spinelli A, Pellino G. COVID-19 pandemic: perspectives on an unfolding crisis. Br J Surg. 2020.
- Fauci AS, Lane HC, Redfield RR. Covid-19 â?? navigating the uncharted. N Engl J Med. 2020; 382:1268-1269.
- Mahase E, Kmietowicz Z. Covid-19: doctors are told not to perform CPR on patients in cardiac arrest. BMJ 2020; 368: m1282-m1282.
- Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, et al. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis. 2020.
- Weiss P, Murdoch DR. Clinical course and mortality risk of severe COVID-19. Lancet 2020; 395:1014-1015.
- Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020.
- Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020.
- Poston JT, Patel BK, Davis AM. Management of critically ill adults with COVID-19. JAMA. 2020.
- Cao B, Wang Y, Wen D, et al. A trial of lopinavirâ??ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020; 382:1787-1799.
- Shen C, Wang Z, Zhao F, et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020.
- Touret F, de Lamballerie X. Of chloroquine and COVID-19. Antiviral Res 2020.
- Baden LR, Rubin EJ. Covid-19 â?? the search for effective therapy. N Engl J Med. 2020; 382:1851-1852.
- Lo MK, Jordan R, Arvey A, et al. GS-5734 and its parent nucleoside analog inhibit filo-, pneumo-, and paramyxoviruses. Sci Rep. 2017; 7: 43395.
- WHO. WHO R&D blueprint: informal consultation on prioritization of candidate therapeutic agents for use in novel coronavirus 2019 infection.Geneva: WHO; 2020.
- de Wit E, Feldmann F, Cronin J, et al. Prophylactic and therapeutic Remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc Natl Acad Sci U S A 2020; 117: 6771-6776.
- Sheahan TP, Sims AC, Graham RL, et al. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci Transl Med 2017; 9(396): eaal3653-eaal3653.
- WHO Solidarity Trial Consortium. Repurposed antiviral drugs for covid-19â??interim WHO Solidarity trial results. 2020.
- Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020.
- Grein j, Ohmagari N, Shin D, Diaz G, Asperges E, Castagna A et al. Compassionate Use of Remdesivir. for Patients with Severe Covid-19.N Engl J Med 2020; 382: 2327-36.
- Poston JT, Patel BK, Davis AM. Management of critically ill adults with COVID-19. JAMA. 2020.
- Cao B, Wang Y, Wen D, et al. A trial of lopinavirâ??ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020; 382:1787-1799.
- Garibaldi BT, Wang K, Robinson ML, Zeger SL, Roche KB, Wang MC. Comparison of Time to Clinical Improvement With vs Without Remdesivir Treatment in Hospitalized Patients With COVID-19.JAMA Network Open. 2021; 4(3):e213071.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.