Ribociclib/Letrozole Combination for the Treatment of Locally Advanced Hr+/Her2-Breast Cancer: A Real-World Experience
Received: 28-Nov-2022, Manuscript No. amhsr-23-87282; Editor assigned: 30-Nov-2022, Pre QC No. amhsr-23-87282 (PQ); Reviewed: 13-Dec-2022 QC No. amhsr-23-87282; Revised: 20-Dec-2022, Manuscript No. amhsr-23-87282 (R); Published: 30-Dec-2022
Citation: Savastano C. Ribociclib plus Letrozole for Locally Advanced Hr+/Her2-Breast Cancer: A Real-World Experience. Ann Med Health Sci Res. 2022;12:38-403
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Locally Advanced Breast Cancer (LABC) is a heterogenous disease not amenable for primary surgery. Neoadjuvant therapy has been shown to improve outcomes in patients with inoperable de novo Hormone Receptor-positive/Human Epidermal Growth factor receptor 2-negative (HR+/HER2-) locally advanced breast cancer.
An Italian retrospective single-center study included 23 de novo HR+/HER2-LABC patients, treated with first-line ribociclib plus letrozole. The clinical response was analyzed both at biological and clinical level. At baseline, 15 (65.2%) women had stage IIIA ductal carcinoma; median Ki-67 expression was 15% (range: 10-80%) and 13 patients (56,5%) had Ki-67 under 20%. After treatment, 68.5% of patients achieved overall response with 1 clinical complete response; 73.9% were eligible for surgery. Following surgery, 88% experienced TNM downstaging and 56% obtained Ki-67 regression. At a median follow-up of 18 months, all patients were alive.
Thus, a Cyclin-Dependent Kinase (CDK) 4/6 inhibitor (ribociclib) in combination with a nonsteroidal aromatase inhibitor (letrozole) represents an effective strategy to reduce tumor volume and to facilitate conservative surgery also in those patients initially considered inoperable. To our knowledge, this is the first real-world observational report of ribociclib plus letrozole in this setting.
Keywords
Ribociclib; Locally advanced breast cancer; Neoadjuvant; Real world
Introduction
Locally Advanced Breast Cancer (LABC) is a heterogenous disease without an univocal definition. It represents an important clinical issue, since it is still not so well characterized from both biological and clinical point of view, so far. Some authors used to consider LABC a disease not amenable for primary surgery, including those tumors of ≥ 5 cm, or involving the chest wall, muscle, or skin, or with satellite nodules or with an extensive nodal involvement [1]. Neoadjuvant chemotherapy is an established multimodality protocol for the treatment of LABC, reducing the tumor burden and increasing the likelihood of R0 resection [2]. The opportunity of a breast conserving surgery and the feasibility to follow individual tumor response are some advantages of this therapy. However, only 1 in 5 patients achieves pathological Complete Response (pCR), a surrogate endpoint associated to a favorable prognosis. In addition, resistance and safety issues may occur, so that research for alternatives is needed, especially in elderly and frail patients. To overcome this issue, authors evaluated various lines of evidence from previous studies which highlighted the role of the combination of the neoadjuvant treatment with an Endocrine Therapy (ET), the backbone of HR+/HER2-advanced BC treatment [3]. Basically, endocrine therapy strategies are based on the inhibition of the production of estrogen, by using Aromatase Inhibitors (AI) or by directly targeting estrogen receptor [4]. Several clinical trials extensively investigated the administration of CDK4/6 inhibitors as neoadjuvant drug in patients with HR+/ HER2-negative genomic profile. Addition of selective cyclin- dependent kinase 4/6 inhibitors, like palbociclib, ribociclib and abemaciclib, to ET has provided remarkable results in patients with distance metastasis [3,5–12]. Ribociclib has shown a good activity in the treatment of metastatic breast cancer in peri/ premenopausal women. The small molecule is given per os and plays an important role in regulating cell cycle progression and cellular proliferation. The combination of CDK inhibitors plus ET displayed a similar efficacy with a more favorable safety profile, compared to chemotherapy alone [13]. Albeit most are single-arm phase 2 trials with limited sample size, preliminary findings showed high antiproliferative effect (as measured by Ki-67 levels<2.7% (i.e., complete cell cycle arrest, CCCA) and a good safety profile [14].
Here we present data from a neoadjuvant therapy, ribociclib plus letrozole, used as a first-line treatment in patients presenting inoperable de novo HR+/HER2-LABC without evidence of distant metastases.
Patients and Methods
The present Italian retrospective single-center study enrolled twenty-three women between September 2018 and January 2021.
The patients included in the study fulfilled the following eligibility criteria: (i) diagnosed with HR+/HER2-LABC; (ii) not amenable for surgery; (iii) aged ≥ 18 years; (iv) in pre-e post-menopausal condition. Patients were treated with ribociclib 600 mg/die (3 weeks on/1 week off) plus letrozole (2.5 mg/die continuously) upfront. Pre-menopausal women received also goserelin. After surgery, patients continued endocrine therapy alone as adjuvant therapy [15].
All procedures were performed in accordance with the principles of the Declaration of Helsinki and with the regulations and guidelines governing medical practice and ethics in Italy. All patients provided written informed consent before participating in the study.
Assessments
Clinical and radiological evaluations were performed every 12 weeks. Radiological response was assessed with ultrasound, resonance and computed tomography scan according to the Response Evaluation Criteria in Solid Tumors 1.1 (RECIST 1.1) criteria [16].
Estrogen Receptor (ER), Progesterone Receptor (PgR), HER2 and Ki-67 were classified on the basis of the St. Gallen and American Society of Clinical Oncology-College of American Pathologists guidelines [17]. A cut-off of positively stained cells of 20% was used to classify the expression of Ki-67 and PgR as high (≥ 20%) or low (<20%) [18]. Luminal cases were classified as A if PgR was ≥ 20% and Ki-67 <20% or B if PgR was <20% and/ or Ki-67 was ≥ 20%. HER2 was assessed using the HercepTest (DAKO Corporation, Carpinteria, CA, USA): Samples scored as 3+ were considered HER2+. In case of equivocal results (2+), we performed fluorescence in situ hybridization.
Toxicities were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0[19].
Statistical analyses
For Ki-67 regression, a Chi-square test for univariate dichotomous variables was performed. Other clinical and pathological variables were presented as descriptive analysis. Data were expressed as frequencies (n (%)), median (range), and mean ± Standard Deviation (SD).
Results
A total of 23 patients were treated with ribociclib plus letrozole. Baseline characteristics of patients are the following: (i) median age was 58 years (range 41-88); (ii) 16 (69.6%) women were post- menopausal; (iii) 15 (65.2%) had stage IIIA ductal carcinoma; (iv) 7 (69.6%) reported a complex nipple-areola abnormality.
The median level of specific biomarkers was measured. ER, PgR and Ki-67 expression was 90% (range: 70%-90%), 80% (range: 0%-90%) and 15% (range: 10%-80%), respectively. 14 patients (60.9%) had luminal B LABC. All these informations are summarized in Table 1.
| Parameter | Baseline | After treatment (pharmacological and surgical) |
|---|---|---|
| Age, years | 58 (41-88) | |
| Menopausal status | ||
| Pre | 7 (30.4%) | |
| Post | 16 (69.6%) | |
| Histology | ||
| Ductal/NST carcinoma | 22 (95.7%) | |
| Lobular | 1 (4.3%) | |
| Tumor size | (n=23) | (n=16) |
| T1 | 3 (13.0%) | 10 (62.5%) |
| T2 | 9 (39.1%) | 5 (31.3%) |
| T3 | 5 (21.7%) | 1 (6.3%) |
| T4 | 6 (26.1%) | 0 (0.0%) |
| Nodal status | (n=23) | (n=16) |
| N0 | 0 (0.0%) | 6 (37.5%) |
| N1 | 5 (21.7%) | 8 (50.0%) |
| N2 | 18 (78.3%) | 2 (12.5%) |
| N3 | 0 (0.0%) | 0 (0.0%) |
| Clinical Stage | (n=23) | (n=17) |
| I | 0 (0%) | 6 (34.0%) |
| IIA | 0 (0%) | 6 (37.5%) |
| IIB | 2 (8.7%) | 3 (18.7%) |
| IIIA | 15 (65.2%) | 2 (12.5%) |
| IIIB | 6 (26.1%) | 0 (0.0%) |
| Grading, | (n=20) | (n=16) |
| G1 | 3 (15.0%) | 6 (37.5%) |
| G2 | 10 (50.0%) | 7 (43.8%) |
| G3 | 7 (35.0%) | 3 (18.8%) |
| Ki-67 expression | (n=23) | (n=16) |
| <20% | 13 (56.5%) | 14 (87.5%) |
| ≥ 20% | 10 (43.5%) | 2 (12.5%) |
| HR expression | (n=23) | (n=16) |
| ER, median (range) | 90 (70-90) | 90 (70-95) |
| 1-89% | 3 (13.0%) | 7 (43.8%) |
| ≥ 90% | 20 (87.0%) | 9 (56.3%) |
| PgR, median (range) | 80 (0-90) | 0 (0-80) |
| 0% | 2 (8.7%) | 11 (68.8%) |
| 1-19% | 3 (13.0%) | 2 (12.5%) |
| ≥ 20% | 18 (78.3%) | 3 (18.7%) |
| Luminal | (n=23) | |
| A | 9 (39.1%) | |
| B | 14 (60.9%) | |
| Multifocality | (n=23) | |
| Unifocal | 20 (87.0%) | |
| Multifocal | 3 (13.0%) | |
| Complex nipple-areola abnormalities | (n=23) | |
| Yes | 7 (69.6%) | |
| No | 16 (30.4%) | |
| Radiological Response | (n=19) | |
| CR | 1 (5.3%) | |
| PR | 12 (63.2%) | |
| SD | 6 (31.6%) | |
| Surgery | (n=16) | |
| Conservative | 1 (6.3%) | |
| Radical | 15 (93.8%) | |
| Adjuvant Radiotherapy | (n=18) | |
| Yes | 13 (72.2%) | |
| No | 5 (27.8%) | |
| Adjuvant therapy | (n=16) | |
| Chemotherapy | 3 (18.8%) | |
| Endocrine therapy | 13 (81.3%) | |
Unless otherwise specified, data refer to all 23 patients. Baseline and post-treatment features were not available for all patients enrolled. Each subsection specifies the number of patients analyzed; NST: No Special Type, HR: Hormone Receptor; ER: Estrogen Receptor; PgR: Progesterone Receptor; CR: Complete Response; PR: Partial Response; SD: Stable Disease
Table 1: Patient characteristics at baseline and after treatment (pharmacological and surgical). Data are expressed as frequencies (n [%]) or median (range).
Unless otherwise specified, data refer to all 23 patients. Baseline and post-treatment features were not available for all patients enrolled. Each subsection specifies the number of patients analyzed; NST: No Special Type, HR: Hormone Receptor; ER: Estrogen Receptor; PgR: Progesterone Receptor; CR: Complete Response; PR: Partial Response; SD: Stable Disease
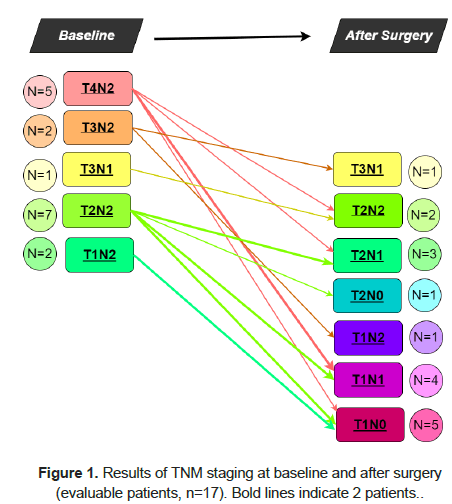
The clinical response in patients was documented with ultrasound, resonance, and computed tomography scan. The median number of treatment cycles, whose range was 1-20, was measured as 6. Overall, 19 (83%) patients were evaluable for clinical response: 13 (68.5%) achieved an objective response (OR, Complete Response–CR + Partial Response-PR), with both clinical and radiological evidence of tumor shrinkage (Table 1). One patient (5.3%) had a clinical CR and refused surgery, while 16 (69.6%) underwent surgery (radical mastectomy with lymph-nodes removal in most cases (93.8%)). The y-TNM stage of disease was I, IIA, IIB and IIIA in 6 (34%), 6 (37%), 3 (19%) and 2 (13%) cases, respectively. Stage IIIB disease was not documented. No pathologic CR (pCR) was reported but, including one patient with a clinical CR, 15 (88%) patients experienced a TNM-stage regression Figure 1.
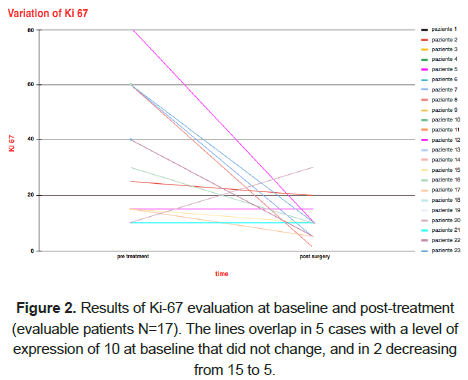
The clinical response was then evaluated also through ImmunoHistoChemistry (IHC) analysis. After surgery, the proportion of Ki-67, ER, and PgR positively stained cells was 10% (range 1%-30%), 90% (range 70%-95%) and 0% (range 0%-80%), respectively. HER2 was negative in all patients. However, patients who experienced Ki-67 regression reported a higher Objective Response Rate (ORR) compared to patients who did not report a Ki-67 regression (77.8% vs. 42.9%, HR=4.66, 95% CI 0.53–40.88, P=0.164) (Figure 2). After performing a Chi-square test for Ki-67 regression, none clinicopathological features showed a significant correlation except for clinical T, even with a trend toward significance (Ki-67 regression was reported in T1-T2 vs. T3-4 in 77.8% vs. 22.2% of cases, P=0.061).
At the time of this analysis, after a median follow-up of 18 months, all patients were alive.
In general, safety results showed that treatment with ribociclib and letrozole was well tolerated. The most common grade 3 adverse events were neutropenia and leukopenia: For 6 (26%) patients, the interval between cycles was in median 10 days longer than the standard (i.e., 7 days) to permit hematological recovery. In 1 (4%) patient the dose was reduced to 400 mg/die for grade 2 nausea, and in 1 for prolonged grade 3 neutropenia. Ten (43%) patients did not experience any toxicity.
Discussion
Following the success of ET plus CDK4/6 inhibitors in ABC, the pre-operatory treatment strategy has become very attractive. Hence, we retrospectively analyzed the data of 23 patients with de novo inoperable HR+/HER2-LABC who received ribociclib and letrozole upfront [6]. The ORR was 68.5%, with 1 clinical CR; 73.9% of patients were eligible for surgery. After surgery, 88% of cases displayed TNM downstaging and 56.3% Ki-67 regression. After a median time of 18 months, all patients were alive.
NeoPalAna is a phase 2 single-arm trial with 50 patients stage II-III HR+/HER2-BC that received hormonal therapy with anastrozole for 28 days, followed by the addition of palbociclib for 4 cycles. Serial biopsies at baseline, C1D1, C1D15, and surgery were analyzed for Ki-67, gene expression and mutation profiles. The authors reported as primary endpoint complete cell cycle arrest (CCCA: central Ki-67<2.7%). They observed an CCCA rate significantly higher after adding palbociclib to anastrozole (C1D15 87% vs. C1D1 26%, p<0.001) [20].
In the prospective randomized PALLET trial, were randomized to letrozole monotherapy or letrozole plus palbociclib for 14 wk.
In this trial CDK 4/6 inhibitor palbociclib in combination with letrozole did not increase the clinical response rate compared to letrozole alone. However, malignant cell proliferation measured by Ki-67 expression analyses revealed significant suppression after adding palbociclib to letrozole treatment in the neoadjuvant setting [21].
The optimal duration of treatment with CDK4/6 inhibitors plus hormonal therapy is a much-debated issue because, although prolonging neoadjuvant ET increases the possibility of clinical response and breast conservation, there is no proof that it improves pCR rates. A phase IV clinical trial suggested that neoadjuvant letrozole administered for 7.5 mo was more effective at achieving beneficial shrinkage in tumor volume and facilitating conservative surgery than a 4-mo treatment [22].
In the neoadjuvant setting, ribociclib plus letrozole was tested in two phase 2 studies. MONALEESA-1 included 14 patients and a change in Ki-67 levels was documented in 96% and 92% of patients treated with 400 and 600 mg of ribociclib plus letrozole vs. 69% with letrozole alone. The CORALEEN trial enrolled 106 patients, and at the time of surgery 46.9% of patients treated with ribociclib plus letrozole and 46.1% with chemotherapy attained low-risk-of-relapse disease [23]. Moreover, a complete, long-lasting remission was documented in a patient with de novo ABC with axillary lymph node involvement and both- sided pulmonary metastases treated with ribociclib plus letrozole in first-line, with few side effects and no specific restrictions in daily activities [24]. Before treatment, Ki-67 was strongly expressed in>90% of cells [25]. Although the analysis could not be repeated, the authors speculated that aggressive tumor growth as measured by Ki-67 predict clinical response to ribociclib plus letrozole. In our analysis, the patient which obtained a CR had a Ki-67 expression level of 15% and both patients with higher and lower levels responded to ribociclib therapy. More data are needed to clarify this issue.
Several studies are currently ongoig (PREDIX LumA (NCT02592083) e PREDIX LumB (NCT02603679) NEOLETRIB (NCT05163106) to evaluate CDK4/6 inhibitors plus hormonal therapy as neoadjuvant therapy for LABC. Preliminary results showed that, compared to chemotherapy, palbociclib and abemaciclib provided similar rates of response, cell proliferation and conservative breast surgery, but with a better safety profile. Further investigations are needed to confirm these data and to verify whether this benefit translates into survival advantage.
Conclusion
Although our results are encouraging, the limited sample size and the retrospective design do not allow to draw definitive. To our knowledge, this is the first real-world study of ribociclib plus letrozole in de novo inoperable HR+/HER2-LABC. Ribociclib plus letrozole therapy has proven to be effective and well tolerated, with a high rate of both clinical response and tumor downstaging. Results of the ongoing phase 3 clinical trials with large sample size are awaited.
Acknowledgments
Medical writing and editorial assistance were provided by Clara Ricci, PhD and Maria Vittoria Verga Falzacappa, PhD (EDRA S.p.A., Milan, Italy) and funded by Novartis Farma S.p.A. (Milan, Varese, Italy).
References
- Cardoso F, Costa A, Norton L, Senkus E, Aapro M, et al. ESO-ESMO 2nd international consensus guidelines for Advanced Breast Cancer (ABC2). Ann Oncol. 2014;25:1871-1888.
[Crossref] [Google Scholar] [Indexed]
- Macdonald SM, Harris EE, Arthur DW, Bailey L, Bellon JR, Carey L, et al. ACR appropriateness criteria® locally advanced breast cancer. Breast J. 2011;17:579-585.
[Crossref] [Google Scholar] [Indexed]
- Papadimitriou K, Ardavanis A, Kountourakis P. Neoadjuvant therapy for locally advanced breast cancer: Focus on chemotherapy and biological targeted treatments' armamentarium. J Thorac Dis. 2010;2:160-170.
[Crossref] [Google Scholar] [Indexed]
- Grizzi G, Ghidini M, Botticelli A, Tomasello G, Ghidini A, Grossi F, et al. Strategies for increasing the effectiveness of aromatase inhibitors in locally advanced breast cancer: An evidence-based review on current options. Cancer Manag Res. 2020;12:675-686.
[Crossref] [Google Scholar] [Indexed]
- Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, et al. Ribociclib as first-line therapy for HR-positive, Advanced Breast Cancer. N Engl J Med 2016; 375:1738-1748.
[Crossref] [Google Scholar] [Indexed]
- Piezzo M, Chiodini P, Riemma M, Cocco S, Caputo R, Cianniello D, et al. Progression-free survival and overall survival of cdk 4/6 inhibitors plus endocrine therapy in metastatic breast cancer: A systematic review and meta-analysis. Int J Mol Sci. 2020;21:6400.
[Crossref] [Google Scholar] [Indexed]
- Li J, Huo X, Zhao F, Ren D, Ahmad R, et al. Association of cyclin-dependent kinases 4 and 6 inhibitors with survival in patients with hormone receptor-positive metastatic breast cancer: A systematic review and meta-analysis. JAMA Netw Open. 2020;3:e2020312.
[Crossref] [Google Scholar] [Indexed]
- Finn RS, Boer K, Bondarenko I, Patel R, Pinter T, et al. Overall survival results from the randomized phase 2 study of palbociclib in combination with letrozole versus letrozole alone for first-line treatment of ER+/HER2-advanced breast cancer (PALOMA-1, TRIO-18). Breast Cancer Res Treat. 2020;183:419-428.
[Crossref] [Google Scholar] [Indexed]
- Cristofanilli M, Rugo HS, Im S-A, Slamon DJ, Harbeck N, et al. Overall Survival (OS) with Palbociclib (PAL) + Fulvestrant (FUL) in women with hormone receptor–positive (HR+), human epidermal growth factor receptor 2–negative (HER2–) Advanced Breast Cancer (ABC): Updated analyses from PALOMA-3. JCO. 2021;39:1000-1000.
- Hortobagyi GN, Stemmer SM, Burris HA, Le Gac F, Serra P, et al. LBA17 Overall Survival (OS) results from the phase III MONALEESA-2 (ML-2) trial of postmenopausal patients (pts) with hormone receptor positive/human epidermal growth factor receptor 2 negative (HR+/HER2−) Advanced Breast Cancer (ABC) treated with Endocrine Therapy (ET) ± Ribociclib (RIB). Ann Oncol. 2021;32:S1290-S1291.
- Slamon DJ, Neven P, Chia SKL, Fasching PA, De Laurentiis M, et al. Updated Overall Survival (OS) results from the phase III MONALEESA-3 trial of Postmenopausal Patients (pts) with HR+/HER2-Advanced Breast Cancer (ABC) treated with Fulvestrant (FUL) ± Ribociclib (RIB). JCO. 2021;39:1001-1001.
- Sledge GW Jr, Toi M, Neven P, Sohn J, Inoue K, et al. The Effect of Abemaciclib Plus Fulvestrant on Overall Survival in Hormone Receptor–Positive, ERBB2-Negative Breast Cancer That Progressed on Endocrine Therapy—MONARCH 2: A Randomized Clinical Trial. JAMA Oncology. 2020;6:116-124.
[Crossref] [Google Scholar] [Indexed]
- Syed YY. Ribociclib: First global approval. Drugs. 2017; 77:799-807.
[Crossref] [Google Scholar] [Indexed]
- Spring LM, Gupta A, Reynolds KL, Gadd MA, Ellisen LW, Isakoff SJ, et al. Neoadjuvant endocrine therapy for estrogen receptor-positive breast cancer: A systematic review and meta-analysis. JAMA Oncol. 2016;2:1477-1486.
[Crossref] [Google Scholar] [Indexed]
- ANNEX I. Summary of product characteristics. 2021.
- RECIST 1.1. RECIST. 2021.
- Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, et al. Personalizing the treatment of women with early breast cancer: Highlights of the st gallen international expert consensus on the primary therapy of early breast Cancer 2013. Ann Oncol. 2013;24:2206-2223.
[Crossref] [Google Scholar] [Indexed]
- Harris L, Fritsche H, Mennel R, Norton L, Ravdin P, et al. American society of clinical oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007;25:5287-5312.
[Crossref] [Google Scholar] [Indexed]
- U.S.Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE). 2009.
- Ma CX, Gao F, Luo J, Northfelt DW, Goetz M, Forero A, et al. Neopalana: Neoadjuvant palbociclib, a cyclin-dependent kinase 4/6 inhibitor, and anastrozole for clinical stage 2 or 3 estrogen receptor-positive breast cancer. Clin Cancer Res. 2017;23:4055-4065.
[Crossref] [Google Scholar] [Indexed]
- Johnston S, Puhalla S, Wheatley D, Ring A, Barry P, Holcombe C, et al. Randomized phase II study evaluating palbociclib in addition to letrozole as neoadjuvant therapy in estrogen receptor-positive early breast cancer: PALLET Trial. J Clin Oncol. 2019;37:178-189.
[Crossref] [Google Scholar] [Indexed]
- Fontein DB, Charehbili A, Nortier JW, Kranenbarg EMK, Kroep JR, Putter H, et al. Efficacy of six month neoadjuvant endocrine therapy in postmenopausal, hormone receptor-positive breast cancer patients-a phase II trial. Eur J Cancer. 2014;50:2190-2200.
[Crossref] [Google Scholar] [Indexed]
- Curigliano G, Gómez Pardo P, Meric-Bernstam F, Conte P, Lolkema MP, Beck JT, et al. Ribociclib plus letrozole in early breast cancer: A presurgical, window-of-opportunity study. Breast. 2016;28:191-198.
[Crossref] [Google Scholar] [Indexed]
- Prat A, Saura C, Pascual T, Hernando C, Muñoz M, Paré L, et al. Ribociclib plus letrozole versus chemotherapy for postmenopausal women with hormone receptor-positive, HER2-negative, luminal B breast cancer (CORALLEEN): An open-label, multicentre, randomised, phase 2 trial. Lancet Oncol. 2020;21:33-43.
[Crossref] [Google Scholar] [Indexed]
- Rudlowski C, Beermann N, Leitzen L, Nuding B. Complete clinical response in locally advanced metastatic de novo breast cancer after front-line treatment with ribociclib/letrozole within the RIBANNA study. Breast Care (Basel). 2020;15:289-293.
[Crossref] [Google Scholar] [Indexed]




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.