Risk of Intracranial Hemorrhage and Utilization of Selective Serotonin Reuptake Inhibitors: Systematic Review and Meta-analysis
Medical Intern, Umm AlQura University, Makkah City, Saudi Arabia
2 Medical Intern, Dar AlUloom University, Riyadh City, Saudi Arabia
3 General Practitioner, University of Jeddah, Jeddah City, Saudi Arabia
4 Medical Intern, King Faisal University, Alahsa City, Saudi Arabia
Citation: Alsayat JY, et al. Risk of Intracranial Hemorrhage and Utilization of Selective Serotonin Reuptake Inhibitors: A Systematic Review and Meta-analysis. Ann Med Health Sci Res. 2021;11: 1228-1231.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Background: Selective serotonin reuptake inhibitors (SSRIs) have the potential to reason an obtained hemostatic defect related to their capacity to inhibit platelet characteristics. Especially, SSRIs inhibit the serotonin transporter on platelets, causing a lower in intra platelet serotonin and diminished remarks activation at some point of dense granule secretion, leading to intracranial hemorrhage (ICH). Aim: This work aims to determine the safety of utilization of Selective Serotonin Re-uptake Inhibitors (SSRI), regarding the incidence of Intra-cranial hemorrhage (ICH). Materials and Methods: A systematic search was performed over different medical databases to identify Neurology studies, which studied the outcome of the SSRI users group versus the Non-SSRI users' group of patients. Using the meta-analysis process, either with fixed or random-effects models, we conducted a meta-analysis on the incidence of intracranial hemorrhage as the main primary outcome. Results: Seven studies were identified involving 153323 patients, with 19010 patients in the SSRI users group, and 134313 patients in the Non-SSRI users' group. The meta-analysis process revealed a significant increase in the incidence of intracranial hemorrhage in the SSRI users group compared to the Non-SSRI users' group (p = 0.033). Conclusion: To conclude, SSRIs exposure after ICH is associated with both improvement in depressive symptoms and increased risk of recurrent hemorrhagic stroke. Clinical history, neuroimaging data, and genetic biomarkers may help to identify survivors of ICH more likely to safely tolerate SSRI use.
Keywords
Intracranial Hemorrhage; Selective Serotonin Reuptake Inhibitors (SSRI)
Introduction
The acute presentation of intracranial hemorrhage ICH can be difficult to differentiate from ischemic stroke. Signs and symptoms may additionally consist of headache, nausea, seizures, and focal or generalized neurologic signs. Findings which include coma, headache, vomiting, seizures, and neck stiffness, and raised diastolic blood strain growth the chance of ICH compared to ischemic stroke, but only neuroimaging can provide a definitive prognosis. Primary ICH is commonly a manifestation of underlying small vessel disease. First, longstanding high blood pressure results in hypertensive vasculopathy causing microscopic degenerative adjustments within the partitions of small-to-medium penetrating vessels, which is called lipohyalinosis. CAA is characterized by the deposition of amyloid-beta peptide (Aβ) inside the partitions of small leptomeningeal and cortical vessels. Although the underlying mechanism leading to the accumulation of amyloid remains unknown, the very last outcomes are degenerative adjustments inside the vessel wall characterized by the lack of smooth muscle cells, wall thickening, luminal narrowing, microaneurysm formation, and microhemorrhages. [1]
Selective serotonin reuptake inhibitors (SSRIs) are commonly encountered inside the perioperative period because they are widely used to deal with some fairly ordinary sicknesses, inclusive of depression, anxiety, panic sickness, obsessivecompulsive sickness, bulimia nervosa, and neuropathic ache. SSRIs have the potential to reason an obtained hemostatic defect related to their capacity to inhibit platelet characteristics. Especially, SSRIs inhibit the serotonin transporter on platelets, causing a lower in intra platelet serotonin and diminished remarks activation at some point of dense granule secretion, leading to intracranial hemorrhage (ICH). [2]
SSRI use may additionally affect ICH results. In ischemic stroke, pre-stroke SSRI use has been related to elevated 30- day mortality and decrease prices of discharge to home, but these findings have now not been reproduced. Current clinical trials located inconsistent efficacy of fluoxetine initiation after ischemic stroke for lengthy-time period motor healing. but, little data exist to guide SSRI use in ICH. [3]
This work aims to determine the safety of utilization of Selective Serotonin Reuptake Inhibitors (SSRI), regarding the incidence of intracranial hemorrhage.
Literature Review
Our review came following the (PRISMA) statement guidelines. [4]
Study eligibility
The included studies should be in English, a journal published article, and a human study describing intra-cranial hemorrhage patients. The excluded studies were non-English or animal studies.
Study identification
Basic searching was done over the PubMed, Cochrane library, and Google scholar using the following keywords: Intracranial Hemorrhage, Selective Serotonin Reuptake Inhibitors.
Data extraction and synthesis
RCTs, clinical trials, and comparative studies, which studied the outcome of the SSRI users group versus the Non-SSRI users’ group of patients, will be reviewed. Outcome measures included the incidence of intracranial hemorrhage as the main primary outcome.
Study selection
We found 211 records, 106 excluded because of the title; 105 articles are searched for eligibility by full-text review; 56 articles cannot be accessed; 23 studies were reviews and case reports; 7 were not describing functional outcome; the desired drug not used in 12 studies leaving 7 studies that met all inclusion criteria.
Statistical analysis
Pooled odds ratios (OR), Proportions (%), with 95% confidence intervals (CI) assessed, using a statistical package (MedCalc, Belgium). The meta-analysis process was established via I2- statistics (either the fixed-effects model or the random-effects model), according to the Q test for heterogeneity.
Results
The included studies were published between 2002 and 2020. Regarding the type of included studies, 7 studies (out of 8 studies) were case-control studies, while 1 study was a cohort study [Table 1]. [3,5-10]
Regarding patients’ characteristics, the total number of patients in all the included studies was 153323 patients, with 19010 patients in the SSRI users group, and 134313 patients in the Non-SSRI users’ group, with an average age of (66.8) years [Table 1].
| N | Author | Type of study |
Number of patients | Age (average years) |
||
|---|---|---|---|---|---|---|
| Total | SSRI users group | Non-SSRI users group | ||||
| 1 | Bak et al. [5] | Case-control | 44765 | 2915 | 41850 | 66.8 |
| 2 | Kharofa et al. [6] | Case-control | 2441 | 229 | 2212 | -- |
| 3 | Huhtakangas et al. [7] | Case-control | 676 | 38 | 638 | 66 |
| 4 | Renoux et al. [8] | Case-control | 92738 | 14929 | 77809 | 66.6 |
| 5 | Scheitz et al. [9] | Case-control | 6242 | 266 | 5976 | 70.1 |
| 6 | Kubiszewski et al. [10] | Cohort | 1279 | 281 | 998 | 71.3 |
| 7 | Liu et al. [3] | Case-control | 5182 | 352 | 4830 | 60.5 |
| #Studies arranged via publication year. | ||||||
Table 1: Patients and study characteristics.
A meta-analysis study was done on 7 studies that described and compared the 2 different groups of patients; with an overall number of patients (N=153323) [Table 2]. [3,5-10]
| N | Author | Primary outcome | |
|---|---|---|---|
| Intracranial hemorrhage | |||
| SSRI users group | Non-SSRI users group | ||
| 1 | Bak et al. [5] | 515 | 4250 |
| 2 | Kharofa et al. [6] | 71 | 845 |
| 3 | Huhtakangas et al. [7] | 7 | 51 |
| 4 | Renoux et al. [8] | 588 | 2448 |
| 5 | Scheitz et al. [9] | 13 | 253 |
| 6 | Kubiszewski et al. [10] | 36 | 40 |
| 7 | Liu et al. [3] | 146 | 2141 |
Table 2: Summary of outcome measures in all studies.
Each outcome was measured by:
Odds Ratio (OR)
• For the incidence of intracranial hemorrhage.
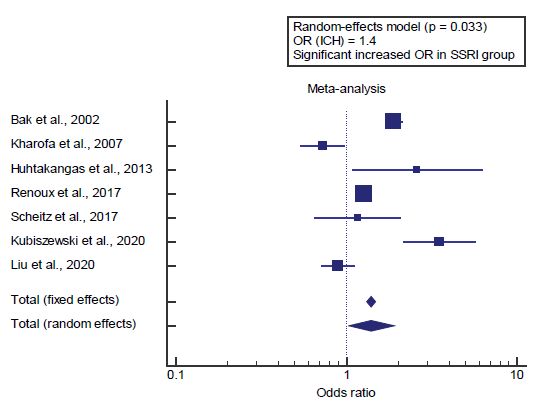
Concerning the primary outcome measure, we found 7 studies reported the incidence of intracranial hemorrhage. I2 (inconsistency) was 93.5%, Q test for heterogeneity (p<0.001), so random-effects model was carried out; with overall OR= 1.4 (95% CI=1.027 to 1.912) [Figure 1].
Using the fixed-effects model, the meta-analysis process revealed a significant increase in the incidence of intracranial hemorrhage in the SSRI users group compared to the Non-SSRI users’ group (p=0.033) [Figure 2].
Discussion
This work aims to determine the safety of utilization of Selective Serotonin Reuptake Inhibitors (SSRI), regarding the incidence of intracranial hemorrhage.
The included studies were published between 2002 and 2020. Regarding the type of included studies, 7 studies (out of 8 studies) were case-control studies, while 1 study was a cohort study.
Regarding patients’ characteristics, the total number of patients in all the included studies was 153323 patients, with 19010 patients in the SSRI users group, and 134313 patients in the Non-SSRI users’ group, with an average age of (66.8) years.
A meta-analysis study was done on 7 studies that described and compared the 2 different groups of patients; with an overall number of patients (N=153323)
Concerning the primary outcome measure, we found 7 studies reported the incidence of intracranial hemorrhage. Using the fixed-effects model, the meta-analysis process revealed a significant increase in the incidence of intracranial hemorrhage in the SSRI users group compared to the Non-SSRI users’ group (p=0.033), which came in agreement with Chollet et al., [11] Hackam & Mrkobrada, [12] Qureshi et al., [13] Teoh et al., [14] Izak & Bussel, [15] Catangui, [16] Kubiszewski et al., [10] Liu et al. [3] and Tully et al. [17]
Chollet et al. reported that, as serotonin is involved in platelet aggregation, a possible increased risk of bleeding complications, including intracerebral bleeding, probably as a result of inhibition of platelet aggregation. [11]
Qureshi et al. reported that Intracranial hemorrhage was related to SSRI exposure in bot unadjusted (RR 1.48) and changed analyses (RR 1.51). Intracerebral hemorrhage became additionally related to SSRI exposure in each unadjusted (RR 1.68) and adjusted (RR 1.42) analysis. In a subset of 5 research (three of intracranial hemorrhage and 1 each reporting hemorrhagic stroke and intracerebral hemorrhage), SSRI publicity in combination with oral anticoagulants was related to an accelerated hazard of bleeding in comparison with oral anticoagulants alone (RR 1.56). [12]
Hackam & Mrkobrada reported that, of their affected person with reversible cerebral vasoconstriction syndrome (RCVS) syndrome, providing with thunderclap-like headache, there is a possibility to be quite simply confused with a migraine. Initiating treatment with selective serotonin reuptake inhibitors (SSRIs) and triptans can similarly worsen the situation. consequently, it’s far vital to understand the nature and form of headache and correlate the medical findings with imaging studies. [13]
Teoh et al. reported that there’s growing literature that associates serotonergic antidepressants with an improved hazard of bleeding. SSRIs have been related to oral adverse results relating to bleeding, consisting of prolonged bleeding time, petechiae, ecchymosis, bruising and gingival bleeding. The proposed mechanism by which this happens is via the inhibition of serotonin reuptake into platelets. Serotonin, in conjunction with other factors along with ADP and prothrombin, potentiates platelet aggregation. [14]
Izak & Bussel reported that the list of medications interfering with platelet function includes beta-lactam antibiotics, nitrates, beta-blockers, tricyclic antidepressants, selective serotonin reuptake inhibitors (SSRIs) and others, most of them only in vitro, having unclear clinical significance, that increase intracranial hemorrhage. [15]
Catangui reported that Selective serotonin reuptake inhibitors or serotonin-specific reuptake inhibitors (SSRIs) and serotoninnorepinephrine reuptake inhibitors (SNRIs) increased the risk of bleeding. [16]
Kubiszewski et al. reported that the mean age of study contributors was 71.3 years, with 602 girls (47%); of the 1279 participants, 1049 had been White, 89 had been Black, 77 were Hispanic, and 64 have been of other race/ethnicity. SSRI exposure became related to each ICH recurrence (RR, 1.31) and resolution of publish-ICH depression (RR, 1.53). amongst those individuals at high threat for recurrent ICH, SSRIs have been related to further elevation in threat for ICH recurrence (RR, 1.79) in comparison with all different survivors of ICH (RR, 1.20; P<0.01 for a contrast of effect sizes). The association of SSRI with reduced depressive signs and symptoms did not differ between excessive the ones at excessive threat for recurrent ICH and all other ICH survivors. [10]
Liu et al. reported that the cohort consisted of 2287 ICH cases and 2895 controls. Pre-ICH SSRI use became now not related to ICH danger (OR, 0.824) nor potentiation of ICH danger with anticoagulant or antiplatelet use. New publish-ICH SSRI use changed into related to unfavorable modified Rankin Scale score at 3 months after ICH (OR, 1.67 P<0.01) in multivariable analyses. Extra propensity rating evaluation indicated a comparable trend
but did not reach statistical significance (P>005). [3]
Tully et al. reported that the study suggested that antidepressant use for<6 months increased stroke risk, while spontaneous intracranial hemorrhage is a risk among SSRI users particularly in the first 30 days of use and when used concomitantly with oral anticoagulants. [17]
Conclusion
To conclude, SSRIs exposure after ICH is associated with both improvement in depressive symptoms and increased risk of recurrent hemorrhagic stroke. Clinical history, neuroimaging data, and genetic biomarkers may help to identify survivors of ICH more likely to safely tolerate SSRI use.
Competing Interests
The authors declare that they have no competing interests. All the listed authors contributed significantly to the conception and design of study, acquisition, analysis, and interpretation of data and drafting of the manuscript, to justify authorship.
REFERENCES
- Caceres JA, Goldstein JN. Intracranial hemorrhage. Emergency Medicine Clinics of North America 2012;30:771.
- Sajan F, Conte JV, Tamargo RJ, Riley LH, Rock P, Faraday N. Association of selective serotonin reuptake inhibitors with transfusion in surgical patients. Anesthesia & Analgesia 2016;123:21–8.
- Liu L, Fuller M, Behymer TP, Ng Y, Christianson T, Shah S, et al. Selective Serotonin Reuptake Inhibitors and Intracerebral Hemorrhage Risk and Outcome. Stroke 2020;51:1135–1141.
- Liberati A, Altman D, Tetzlaff J, Mulrow C, Gøtzsche P, Ioannidis J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions. Bmj 2009;339.
- Bak S, Tsiropoulos I, Kjærsgaard JO, Andersen M, Mellerup E, Hallas J, et al. Selective serotonin reuptake inhibitors and the risk of stroke: a population-based case-control study. Stroke 2002;33:1465–1473.
- Kharofa J, Sekar P, Haverbusch M, Moomaw C, Flaherty M, Kissela B, et al. Selective serotonin reuptake inhibitors and risk of hemorrhagic stroke. Stroke 2007;38:3049–3051.
- Huhtakangas J, Löppönen P, Tetri S, Juvela S, Saloheimo P, Bode MK, et al. Predictors for recurrent primary intracerebral hemorrhage: a retrospective population-based study. Stroke 2013;44:585–590.
- Renoux C, Vahey S, Dell’Aniello S, Boivin J-F. Association of selective serotonin reuptake inhibitors with the risk for spontaneous intracranial hemorrhage. JAMA Neurology 2017;74:173–180.
- Scheitz JF, Turc G, Kujala L, Polymeris AA, Heldner MR, Zonneveld TP, et al. Intracerebral hemorrhage and outcome after thrombolysis in stroke patients using selective serotonin-reuptake inhibitors. Stroke 2017;48:3239–3244.
- Kubiszewski P, Sugita L, Kourkoulis C, DiPucchio Z, Schwab K, Anderson CD, et al. Association of Selective Serotonin Reuptake Inhibitor Use After Intracerebral Hemorrhage With Hemorrhage Recurrence and Depression Severity. JAMA Neurology 2020.
- Chollet F, Tardy J, Pariente J. The Role of Fluoxetine and Selective Serotonin Re-uptake Inhibitors in Motor Recovery Following Acute Ischemic Stroke. Trials 2012;40:80.
- Hackam DG, Mrkobrada M. Selective serotonin reuptake inhibitors and brain hemorrhage: a meta-analysis. Neurology 2012;79:1862–1865.
- Qureshi IA, Qureshi MA, Kanu O, Cruz-Flores S. A 31-year-old with idiopathic reversible cerebral vasoconstriction syndrome. Clinical Case Reports 2017;5:1544–1545.
- Teoh L, Moses G, McCullough MJ. A review of drugs that contribute to bleeding risk in general dental practice. Australian Dental Journal 2020.
- Izak M, Bussel JB. Management of thrombocytopenia. F1000prime Reports 2014;6.
- Catangui EJ. Safe administration of non-vitamin K antagonist oral anticoagulants. Nurse Prescribing 2017;15:238–245.
- Tully PJ, Alpérovitch A, Soumaré A, Mazoyer B, Debette S, Tzourio C. Association Between Cerebral Small Vessel Disease With Antidepressant Use and Depression: 3C Dijon Magnetic Resonance Imaging Study. Stroke 2020;51:402–408.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.