Salivary Albumin as a Biomarker for Oral Squamous Cell Carcinoma and Chronic Periodontitis
Citation: Mallikarjuna Rao Koduru. Salivary Albumin as a Biomarker for Oral Squamous Cell Carcinoma and Chronic Periodontitis. Ann Med Health Sci Res. 2017; 7: 337-340
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Background: Detection of cancer at the early stage is of great importance to decrease the morbidity and mortality of the disease. And as it is known that in neoplastic conditions, many biochemical substances deviate from their normal values; these may include proteins of body fluid. Analysis of salivary markers may provide an economical and noninvasive approach for screening large population. Periodontal infection has been implicated as risk factor for systemic diseases such as coronary heart diseases and diabetes. Aim: The purpose of this preliminary study is to investigate whether salivary albumin levels can be used as an indicator for early detection of oral squamous cell carcinoma and in early detection of chronic periodontitis. Methods: In this cross-sectional study a total of 90 participants were recruited from Dept. of Periodontics, A. B. Shetty Memorial Institute of Dental Sciences and Dept of Oncology, K. S. HEGDE Medical Academy. The participants were divided into three groups of 30 each. Group A -healthy individuals, Group B -patients with chronic generalized periodontitis & Group C -patients with oral squamous cell carcinoma. Salivary Albumin levels were measured using commercially available kits. The difference in the albumin levels between the three groups were compared using Kruskall Wallis test followed by Mann- Whitney U test. P value of less than 0.05 was considered statistically significant. Results: The mean value of Albumin in group A (healthy) is 0.28(SD is 0.19), in group B (chronic gen periodontitis) is 0.31(SD is 0.19), and in group C (OSCC) is 0.82.(SD is 0.41). On comparing the three mean values it is seen that there is a significant rise in salivary levels of Albumin in OSCC patients, but there was no significant rise in salivary albumin levels in chronic periodontitis cases when compared with the healthy group. Conclusion: This study shows that Salivary Albumin levels could be used as a biomarker in Oral squamous cell carcinoma cases but may not be useful as a biomarker for early detection of chronic generalized periodontitis.
Keywords
Oral squamous cell carcinoma; Albumin; Chronic periodontitis
Introduction
Oral squamous cell carcinoma (OSCC) is common and one of the most dreaded types of cancer with high rates of mortality and morbidity all over the world [1]. The increasing incidence of OSCC among women and young and middle-aged males is particularly challenging [2-4]. In India, the incidence rate is high due to consumption of tobacco in various forms, consumption of alcohol, cultural factors, and lack of awareness regarding oral health. Of all cancers in India, the incidence of OSCC is highest in males and third highest in females [5]. Despite advances in surgical and radiation treatments and chemotherapy, the average 5-year overall survival rate of advanced OSCC is not much higher than 50% [6]. Hence detection of OSCC in early stages is very important. Various cytokines, proteins’ and growth factors play a significant role in pathophysiologic regulation of tumor growth and progression.
Chronic periodontitis is considered a multifactorial disease. In the presence of systemic or environmental factors that may modify the host response to plaque accumulation, such as diabetes, smoking, or stress, disease progression may become more aggressive [7]. Chronic Periodontitis is characterized by tissue destruction, bone loss and periodontal attachment loss. Many bacterial components directly and indirectly cause tissue destruction. Along with them mediators such as protienases, cytokines and prostaglandins produced as a part of the host response also contribute to tissue destruction [8].
In the oral cavity albumin is regarded as a serum ultra-filtrate to the mouth, and it may also diffuse into the mucosal secretions. Salivary albumin has been shown to be increased in medically compromised patients whose general condition gets worse. Immunosuppression, radiotherapy, and diabetes are examples of states in which high concentrations of salivary albumin have been detected [9].
Therefore the purpose of this preliminary study is to investigate whether salivary albumin levels can be used as an indicator for early detection of oral squamous cell carcinoma and in early detection of chronic periodontitis.
Materials and Methods
The study protocol was reviewed and approved by the Ethical committee of A.B. Shetty Memorial Institute of Dental Sciences, NITTE University. Signed informed consent was obtained from each participant.
Participant population
Three groups of study participants, all above 18 years of age, were recruited from A.B. Shetty Memorial Institute of Dental Sciences and K.S. Hegde Charitable hospital, Mangalore. (Constituent institutes of NITTE University). The description of each group is as follows. Group A – healthy individuals, Group B – individuals with chronic generalised periodontitis, Group C – individuals newly diagnosed with OSCC (all were either stage III or IV).
Patients with good oral compliance having 20 teeth, with gingival index ≤ 1 were included in group A. Whereas the patients with gingival score 2, pocket depth and clinical attachment loss ≥ 5mm in ≥ 30 percent sites for group B [10]. Pathology reports of OSCC at the time of saliva collection were taken for Group C individuals.
Individuals less than 18 years of age and with any other oral lesion, systemic diseases or with history of any antibiotic/antiinflammatory/ anti-depressant therapy for 3 months prior to study, previous chemotherapy, radiation therapy to head and neck area, subjects who had undergone any periodontal therapy 3 months prior to study were excluded from the study sample.
Screening was done by Medical and dental history of the subjects will be recorded. Gingival index is recorded according to criteria given by Loe and Silness. Clinical Attachment loss has been measured by using Williams graduated periodontal probe.
Saliva sample collection
Saliva samples were collected in the morning between 6 am and 12 pm. Participants were asked not to eat, drink, or perform any kind of oral hygiene procedures before saliva collection. Just before saliva collection, a cup of water was given to the participant for rinsing. Five minutes after rinsing, participants were asked to spit into a 50-mL sterile plastic tube kept in ice. A maximum of 8 ml saliva was collected within 30 minutes.
Saliva sample processing
Immediately after the saliva sample was collected, it was centrifuged at 2,600X g for 20 minutes at 4ºC. After centrifugation, the supernatant was separated from the pellet. Three proteinase inhibitors were added to each 1 ml of the supernatant: 1 ml aprotinin (10 mg/ml), 3 ml sodium ortho vanadate (400 mm), and 10 ml phenyl methyl sulfonyl fluoride (10 mg/ml). The samples were then stored at -80C until future analysis. All samples were analyzed within 6 months after the samples were collected and processed.
Determination of salivary albumin levels
After thawing, Albumin level in the samples was measured by Albumin Agappe kit done by bcg method, and the values were obtained.
Statistical analysis
After thawing, Albumin level in the samples was measured by Albumin Agappe kit done by bcg method, and the values were obtained.
Statistical analysis
The data was entered in Microsoft XL spread sheet and analysed using statistical package for social sciences, version 20 (SPSS 20). Descriptive statistics were presented in terms of mean, standard deviation, medians and quartiles. The difference in the albumin levels between the three groups were compared using Kruskall Wallis test followed by Mann-Whitney U test. P value of less than 0.05 was considered statistically significant.
Results
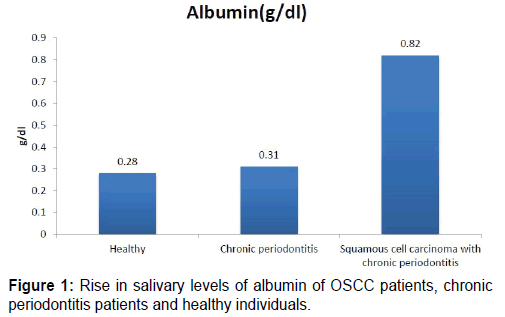
The mean value of Albumin in group A (healthy) is 0.28 (SD is 0.19), in group B (chronic gen periodontitis) is 0.31 (SD is 0.19), and in group C (OSCC) is 0.82 (SD is 0.41). On comparing the mean values of three groups it is seen that there is significant rise in salivary levels of albumin in OSCC patients, but there is no significant rise in salivary levels of albumin in chronic gen periodontitis patients when compared with group of healthy individuals [Table 1 and Figure 1].
| Variables | Mean (SD) | Median (Q1-Q3) | Kruskall Wallis test | Group 1 vs. Group 2 | Group 1 vs. Group 3 | Group2 vs. Group 3 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Chi square value (df) | p-value | U-statistics | p-value | U-statistic | p-value | U-statistics | p-value | ||||
| Albumin (g/dl) | Group 1 | 0.28 (0.19) | 0.27 (0.15-0.33) | 32.84 | <0.001* | 407.5 | 0.53 (NS) | 108.0 | <0.001* | 125.0 | <0.001* |
| Group 2 | 0.31 (0.19) | 0.26 (0.19-0.38) | |||||||||
| Group 3 | 0.82 (0.41) | 0.76 (0.48-1.24) | |||||||||
Group 1 :- Healthy; Group 2 :- Chronic periodontitis; Group 3 :- Squamous cell carcinoma with chronic periodontitis; *P<0.001 statistically significant; p>0.05 non-significant (NS)
Table 1: Comparing the salivary levels of albumin significant values of OSCC patients, chronic periodontitis patients and healthy individuals.
Discussion
The present study is done to know whether levels of salivary Albumin can be used as potential biomarkers for early diagnosis of OSCC and CP detection. The results showed that Salivary Albumin levels could be used as a biomarker in oral squamous cell carcinoma cases but may not be used as a biomarker in chronic periodontitis patients.
Vast number of molecular markers has been correlated with OSCC outcome, illustrating the complex events leading to carcinogenesis and cancer progression. Some of the proposed markers are cytokines like inteleukin-6, interleukin-8, tumor necrosis factor etc.
A study by Mahnaz Saheb Jamee et al. showed that there was a statistically significant rise in salivary interleukin 6 levels in patients with OSCC when compared to healthy individuals. There was also a rise in the salivary tumor necrosis factor, IL-1 and IL-8 in the case group when compared to the control group but it was not statistically significant [11].
In a study by Maie A.R., St. John et al. the salivary and serum levels of IL-6 and IL-8 were assessed. The results showed that IL-8 levels were higher in the saliva (P<0.01) and IL-6 levels were higher in the serum of patients with OSCC (P. 01).
A study was conducted with 19 patients of type-1 and type-2 tongue and gum cancer (9 men and 10 women) and 20 control subjects (15 men and 5 women). IL-1, IL-6, IL-8, and osteopoitin levels in the saliva were assessed with ELISA test. The results showed that the salivary levels of IL-1 and IL-6 were elevated in cancer patients compared to the control group. There was no significant rise in the salivary level of IL-8 and osteopoitin [12].
Another study by Alexis et al. in the tongue of squamous cell carcinoma cases showed that IL-1α, IL-6, IL-8, vascular endothelial growth factor A (VEGF-a), and tumor necrosis factor alpha (TNF-α) levels in the saliva can detect the development and progression of tongue squamous cell carcinoma at an early stage, substantiating their utility as biomarkers for cancer screening and early detection [13].
Similarly numbers of markers were correlated with chronic periodontitis. Some examples are listed below.
Salivary interleukin-1 beta is elevated in periodontal diseases and its levels are strongly correlated with periodontal disease progression and considered to be a good biomarker for discriminating between active and inactive periodontal sites. Salivary interleukin-1 beta has also been associated with advanced periodontitis [14].
According to recent review, interleukin-1 beta and hepatocyte growth factor are the most robust salivary biomarkers for periodontal disease [15]. Increased salivary levels of interleukin-6 are present in individuals with periodontitis compared with healthy subject [16]. Salivary interleukin-6 has also been shown to stimulate osteoclast differentiation and bone resorption and is associated with tissue destruction in peri-implant disease [17].
Salivary levels of tumor necrosis factor-alpha indicate the presence of generalized chronic periodontitis and have also demonstrated significant changes after non-surgical periodontal therapy [18].
As the assessment of salivary levels of cytokines need ELISA kit which is quite expensive, not readily available (can get only on special order) and the kit should be opened only when all the sample size is collected, as the kit expires in a month once opened. Thus it cannot be used when immediate results are needed or when the sample size is small. Whereas salivary albumin levels can be assessed by Albumin Agappe kit this is not expensive and is easily available.
Among the various possible causes of cancer, ROS and reactive nitrogen species found in tobacco rank high as causes of the development of cancer. Plasma levels of antioxidants are reliable indicators of the antioxidant status, because they reflect both bio-availability and increased utilization of antioxidants to counter oxidative stress. Antioxidant levels also are influenced by tobacco and alcohol. Serum proteins have long been known to have antioxidant properties owing to their free thiol groups. Among them, albumin is the most potent and abundant extracellular antioxidant [19].
On the other hand, saliva has long been viewed as a unique, but complex, body fluid. Saliva is the first line of defense against oxidative stress and contains various antioxidants including albumin. The use of saliva as a diagnostic tool has many advantages: it is easy to collect using a noninvasive technique that can be performed at home, no special equipment is required for collection, and it has fewer complications than many alternatives.
Salivary biomarkers have many advantages over serum because they are inexpensive, non-invasive, cost effective and easily accessible media and play a vital role in diagnosis, prediction of prognosis and monitoring of patient’s health [20]. This advantage over other detection methods gives salivary biomarker screening the potential to identify patients with premalignant lesions. Collection is in expensive & can be performed in any settings without the assistance of a health care practitioner. Identifying the proper salivary biomarker profile could serve as a remarkable addition to the current screen method of oral cancer. The ability to identify pre neoplastic states before reaching the stage of invasive disease could have incredible implications for the diagnosis and management of squamous cell carcinoma.
Similarly, salivary biomarker technology has potential to detect recurrence, a common cause of mortality in OSCC patients [21]. Monitoring a patient’s salivary levels post-treatment could be a successful tool for early identification of recurrence.
Conclusion
In this study the salivary albumin levels were raised to a significant level in OSCC subjects, but rise of salivary Albumin levels in chronic periodontitis subjects was not statistically significant.
Based on the results it can be concluded that albumin levels in saliva can be used as biomarker for OSCC but may not use for early detection of chronic periodontitis. More studies will be required to corroborate these findings and verify the possible association between Salivary Albumin and OSCC.
Conflict of Interest
All authors disclose that there was no conflict of interest.
REFERENCES
- Riedel F, Zaiss I, Herzog D, Götte K, Naim R, Hörmann K. Serum levels of Interleukin-6 in patients with primary head and neck squamous cell carcinoma. Anticancer Res 2005;25: 2761-2766.
- SEER Cancer Statistics Review 1975–2007—Previous Version—SEER Cancer Statistics.
- La Vecchia C, Lucchini F, Negri E, Levi F. Trends in oral cancer mortality in Europe. Oral Oncol. 2004;40: 433-439.
- Garavello W, Bertuccio P, Levi F, Lucchini F, Bosetti C, Malvezzi M, et al. The oral cancer epidemic in central and eastern Europe. Int J Cancer. 2010;127: 160-171.
- Byakodi R, Byakodi S, Hiremath S, Byakodi J, Adaki S, Marathe K, et al. Oral cancer in India: An epidemiologic and clinical review. J Community Health 2012;37: 316-319.
- Zhang H, Dziegielewski PT, Biron VL, Szudek J, Al-Qahatani KH, O’Connell DA, et al. Survival outcomes of patients with advanced oral cavity squamous cell carcinoma treated with multimodal therapy: a multi-institutional analysis. J Otolaryngol Head Neck Surg. 2013;42:30
- Newman MG, Takei HH, Klokkevold PR, Carranza FA. Clinical periodontology. (11th edn) South Asia, 2011.
- Newman MG, Takei HH, Klokkevold PR, Carranza FA. Clinical periodontology. (10th edn), South Asia, 2006.
- Meurman JH, Donta DO, Rantonen P, Pajukoski H, Sulkava R. Salivary albumin and other constituents and their relation to oral and general health in the elderly. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2002;94: 432-438.
- Newman MG, Takei HH, Klokkevold PR, Carranza FA. Clinical periodontology: Armitage GC, Classification of periodontal diseases and conditions, (10th edn), 2006.
- Jamee MS, Eslami M, Moghadam FA, Sarafnejad A. Salivary concentration of TNFalpha, IL1 alpha, IL6, and IL8 in oral squamous cell carcinoma. Med Oral Patol Oral Cir Bucal 2008;13: E292-295.
- Katakura A, Kamiyama I, Takano N, Shibahara T, Muramatsu T, Ishihara K, et al. Comparison of salivary cytokine levels in oral cancer patients and healthy subjects. Bull Tokyo Dent Coll 2007;48: 199-203.
- Korostoff A, Reder L, Masood R, Sinha UK. The role of salivary cytokine biomarkers in tongue cancer invasion and mortality. Oral Oncol.2011;47: 282-287.
- Gursoy UK, Kononen E, Pussinen PJ, Tervahartiala T, Hyvarinen K, Suominen AL, et al. Use of host- and bacteria-derived salivary markers in detection of periodontitis: A cumulative approach. Disease Markers.2011;30:299–305.
- Jaedicke KM, Preshaw PM, Taylor JJ. Salivary cytokines as biomarkers of periodontal diseases, Periodontology 2000. 2016; 70, 164-183.
- Costa PP, Trevisan GL, Macedo GO, Palioto DB, Souza SL, Grisi MF, et al. Salivary interleukin-6, matrix metalloproteinase-8, and osteoprotegerin in patients with periodontitis and diabetes, J Periodontol,2010;81: 384-391.
- Liskmann S, Vihalemm T, Salum O, Zilmer K, Fischer K, Zilmer M. Correlations between clinical parameters and interleukin-6 and interleukin-10 levels in saliva from totally edentulous patients with peri-implant disease. Int J Oral Maxillofac Implants. 2006;21: 543-550.
- Sexton WM, Lin Y, Kryscio RJ, Dawson DR III, Miller ECS. Salivary biomarkers of periodontal disease in response to treatment, J Clin Periodontol, 2011;38: 434-441.
- Kaufman E, Lamster IB. 2007. The diagnostic applications of saliva - A review, Crit Rev Oral Biol Med. 13:197-212.
- Radhika T, Nadeem J, Nithya S, Muthumeenakshi RM. Salivary biomarkers in oral squamous cell carcinoma – An insight. J Oral Biol Craniofac Res.2016;6:S51-S54.
- Rusthoven K, Ballonoff A, Raben D, Chen C. Poor prognosis in patients with stage I and II oral tongue squamous cell carcinoma. Cancer 2008;112: 345-351.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.