Spectrum of Thyroid Dysfunction among Patients Evaluated by Thyroid Function Tests at a Tertiary Clinical Laboratory in Calabar, Nigeria
2 Department of Internal Medicine, University of Calabar, University of Calabar Teaching Hospital, Calabar, Nigeria, Email: enang123@gmail.com
3 Department of Family Medicine, University of Calabar, University of Calabar Teaching Hospital, Calabar, Nigeria, Email: aluka123@gmail.com
Citation: Okpara HC, et al. Spectrum of Thyroid Dysfunction among Patients Evaluated by Thyroid Function Tests at a Tertiary Clinical Laboratory in Calabar, Nigeria. Ann Med Health Sci Res. 2017; 7: 411-417
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Background: Thyroid dysfunction is one of the common abnormalities encountered in contemporary clinical endocrinology practice. There is a fairly wide spectrum of thyroid dysfunction which can be identified by patterns of thyroid function test results. Whereas overt hypothyroidism or hyperthyroidism are clinically manifest and can be distinguished by the pattern of changes in serum T4, T3 and TSH levels, subclinical thyroid dysfunctions are essentially laboratory-based diagnoses. Objective: To describe the spectrum (pattern and prevalence) of thyroid dysfunction among patients evaluated by thyroid function tests at a tertiary clinical laboratory. Method: The study was a retrospective study that was carried out by retrieving and reviewing archived thyroid function tests results stored in an electronic database of a fully-automated tertiary clinical laboratory. Results: Among the results, 68.8% indicated euthyriodism while thyroid dysfunction occurred in 31.2% of cases. The spectrum of thyroid dysfunction included: primary hyperthyroidism (13.7%), subclinical hypothyroidism (6.3%), primary hypothyroidism (4.9%), subclinical hyperthyroidism (4.1%), euthyriod sick syndrome (1.5%) and euthyriod hyperthyroxinaemia (0.3%). Primary and subclinical hyperthyroidism were more common in females while primary and subclinical hypothyroidism were more common in males. Conclusion: Thyroid dysfunction is a fairly common endocrine abnormality in our contemporary clinical practice and it occurs in a wide range of spectrum. Primary hyperthyroidism is the most common form of thyroid dysfunction in our environment with women of child-bearing age being the mostly affected.
Keywords
Thyroid function; Thyroid function tests; Thyroid dysfunction
Introduction
Thyroid dysfunction (TD) is one of the common endocrine abnormalities encountered in contemporary clinical endocrinology practice. [1,2] Clinicians commonly request for the laboratory assessment of thyroid function based on the suspicion of thyroid disease in patients with overt or mild clinical features that pertain to thyroid function. Some of the results obtained from the laboratory investigations may be suggestive of clear-cut TD which may be either overt hypothyroidism or hyperthyroidism. [3,4] However, the spectrum of TD based on the pattern of measured thyroid hormones may vary.
While a good number of disorders of thyroid function manifest with clear-cut signs and symptoms, several of them may not be clinically obvious with regard to their presenting symptoms and demonstrable clinical signs. These categories of TD have been referred to as subclinical thyroid dysfunctions. [5,6] Subclinical TD includes both subclinical hypothyroidism and subclinical hyperthyroidism. In both conditions, the measured serum level of both thyroxine (T4) and triodothyronine (T3) remain within health-associated reference limits while serum thyroid stimulating hormone (TSH) concentration is mildly elevated or suppressed respectively. [7,8]
Whereas overt hypothyroidism or hyperthyroidism are clinically manifest and can be distinguished by the pattern of changes in serum T4, T3 and TSH levels, subclinical thyroid dysfunctions are essentially laboratory-based diagnoses. [9] This stems from the fact that in most cases, symptoms of subclinical hypothyroidism and subclinical hyperthyroidism are vague and may not be readily distinguished from other commonplace constitutional symptoms. Thus, the clinical laboratory plays a significant role in establishing the diagnosis of overt thyroid dysfunction, and more especially those of subclinical thyroid dysfunction. [10,11] In addition to overt and subclinical hypothyroidism or hyperthyroidism, other conditions that may be diagnosed by measurement of serum TSH, T3 and T4 include: euthyroid sick syndrome, euthyroid hyperthyroxinaemia, and euthyroid hypothyroxinaemia. [12-14]
Cross river state of Nigeria based on her geographical location, encompasses regions of environmental and nutritional iodine deficiency and sufficiency. [15,16] While the northern region of the state belongs to the mountainous belt with poor soil and food iodine content, the southern region is close to the sea with the potential of environmental iodine sufficiency. Both clinical and epidemiological studies have shown that endemic goiter and its sequalae such as toxic multinodular goiter tend to be more prevalent among individuals living in areas with environmental iodine deficiency especially the women folk. [17-19] In contrast, subclinical hypothyroidism is fairly more frequent in areas of environmental iodine sufficiency. [20]
Based on the above, it can be deduced that TD is a fairly common endocrine disorder in this part of Nigeria. This has prompted the quest to review laboratory test results of thyroid function test at a tertiary clinical laboratory situated in Calabar Municipality of Cross river state. In this laboratory, the assessment of thyroid function is carried out by measurement of serum free or total T3 and T4 as well as serum TSH concentration, all measured using the highly sensitive immuno-chemiluminescent assays. Submitted patients samples are usually processed and analyzed the same day of receiving the sample without preanalytical preservation. Patients’ results were electronically archived in a retrievable database for subsequent reference if the need arises.
The aim of this study was to review the pattern, frequency, ageand gender-distribution of TD using laboratory thyroid function test results retrieved from the electronically archived database of a tertiary clinical laboratory in Calabar, Nigeria.
Materials and Methods
Study location, design and subject selection
The study was carried out at Asiukpo diagnostic and medical centre, Calabar, Cross river state, Nigeria. The centre has a tertiary clinical laboratory that undertakes both routine and specialized biochemical and endocrinological tests, including thyroid function tests. The design of the study was retrospective. It involved the retrieval and review of archived patient’s thyroid function test results from an electronically-stored database. The study protocol was approved by the Ethical Research and Review Committee of the institution.
The patients’ thyroid function test results were selected for the study based on the inclusion and exclusion criteria. The following categories of patients’ results were included in the study viz: (1) all previously performed thyroid function tests results carried out between 1st of January, 2014 to 30th of June, 2015, (2) thyroid function test results of patients between the ages of one day to 79 years. The exclusion criteria included: (1) patients with recorded history of anti-thyroid therapy or thyroid hormone replacement therapy, (2) patients with incomplete thyroid function test profile.
Sampling and data collection
Purposive sampling procedure was used for this study. This involved the enrollment of patients that underwent thyroid function testing between 1st January, 2014 and 30th June, 2015 at the tertiary clinical laboratory.
The sample size for the study consisted of the total number of thyroid function test results of patients that fulfilled the selection criteria. Only thyroid function test results within the period under review were selected. A proforma was designed for collection of data with respect to age and gender of the patients. The thyroid function test results consisting of either serum total thyroxine (TT4) or free thyroxine (FT3), total triodothyronine (TT3) or free tridothyronine (FT3), and thyroid-stimulating hormone (TSH) values were retrieved from the archived database.
Assay methodology for thyroid hormones profile
Serum samples of patients were tested within 1-hour of sample collection using automated immunoassay analyzer. The tests were performed using highly sensitive chemilumnescent immunoassays (CLIA). For all the parameters i.e., TT3, FT3, TT4, FT4, and TSH, both low and high quality control sera were ran together with each batch of patient samples after instrument calibration.
The reference values for the various thyroid function test parameters are given in brackets as follows: TT3 (0.79-1.58 ng/ ml), FT3 (2.1 – 3.8 pg/ml), TT4 (4.9-11.0 ng/ml), FT4 (0.82-1.6 ng/dl) and TSH (0.38-4.31 mIU/L).
Definition and classification of categories of thyroid dysfunction
The categories of TD were classified based on the reference intervals for the hormones and pattern of derangement in the thyroid hormones profile. The abnormal thyroid function tests result was classified into any of the following:
• Subclinical hypothyroidism: Normal TT3 or FT3 and TT4 or FT4 with elevated serum TSH.
• Primary (overt) hypothyroidism: Suppressed serum TT3 or FT3 and TT4 or FT4 with elevated serum TSH.
• Secondary hypothyroidism: Reduced serum concentrations (below lower reference limits) of TT3 or FT3, TT4 or FT4 and TSH.
• Subclinical hyperthyroidism: Normal TT3 or FT3 and TT4 or FT4 with suppressed serum TSH.
• Primary (overt) hyperthyroidism: Elevated serum TT3 or FT3 and TT4 or FT4 with suppressed serum TSH.
• Secondary hyperthyroidism: Elevated serum TT3 or FT3 and TT4 or FT4 and TSH.
• Euthyroid hyperthyroxinaemia: Isolated elevation of FT4 or TT4 in the presence of TSH, FT3 and TT3 within reference limits.
• Euthyroid sick syndrome: Low TT3 or FT3 in the presence of normal TSH and FT4 levels.
Statistical analysis
Data were analyzed to find out the frequencies of various categories of thyroid dysfunction. Data were classified into subgroups according to age and gender so as to determine age-and gender-associated frequency of thyroid dysfunctions. The relative frequencies and ratios of each category of TD were determined. Data were analyzed using SPSS version 16 statistical package. Frequency bar charts and tables were prepared using Microsoft excel software programme. A p-value of < 0.05 was considered statistically significant.
Results
This study was carried out by reviewing thyroid function test results performed between January 2014 and December 2015 (2 years) at the clinical laboratory unit of Asiukpo Medical and Diagnostic Centre located in Calabar, South-south Nigeria. Within the period under review, a total of 441 thyroid function test results were retrieved. Out of this, only 388 (88.0%) results were selected in line with the inclusion and exclusion criteria.
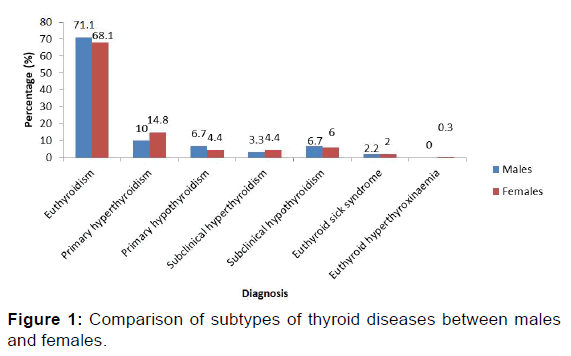
Table 1 shows the general summary of the thyroid function test results and the percentage frequencies of various forms of thyroid function status. Among the results, 68.8% indicated euthyroidism, while TD occurred in 31.2% of cases. The spectrum of TD included: primary hyperthyroidism (13.7%), subclinical hypothyroidism (6.3%), primary hypothyroidism (4.9%), subclincal hyperthyroidism (4.1%), euthyroid sick syndrome (1.5%) and euthyroid hyperthyroxinaemia (0.3%). The study showed that primary hyperthyroidism was the commonest form of TD with a prevalence of 13.7%.
| Diagnosis | Males Frequency (%) |
Females Frequency (%) |
Total Frequency (%) |
Chi square test | p-value |
|---|---|---|---|---|---|
| Euthyroidism | 64 (16.5) | 203 (52.3) | 267 (68.8) | 2.792 | 0.835 |
| Primary hyperthyroidism | 9 (2.3) | 44 (11.3) | 53 (13.7) | ||
| Primary hypothyroidism | 6 (1.5) | 13 (3.4) | 19 (4.9) | ||
| Subclinical hyperthyroidism | 3 (0.8) | 13 (3.4) | 16 (4.1) | ||
| Subclinical hypothyroidism | 6 (1.5) | 18 (4.6) | 24 (6.3) | ||
| Euthyroid sick syndrome | 2 (0.5) | 6 (1.5) | 8 (2.1) | ||
| Euthyroid hyperthyroxinaemia | 0 (0.0) | 1 (0.3) | 1 (0.3) | ||
| Total | 90 (23.2) | 298 (76.8) | 388 (100.0) |
Table 1: Spectrum of thyroid diseases among males and females (n=388).
Figure 1 shows the comparison of thyroid function test results between males and females. Primary hyperthyroidism and subclinical hyperthyroidism occurred more in females than in males while primary and subclinical hypothyroidism were more common in males.
Table 2 represents an age-distribution of the thyroid function test results. Generally, there is a direct relationship between age and the occurrence of TD. Higher prevalence rates of TD were found among patients aged 41 and above (50.0%) followed by those between 16 and 40 years 45.9% and 4.1% among those aged 15 years and below. The difference in the frequency of TD among age groups is statistically significant (p<0.001).
| Age groups (years) | |||||
| Diagnosis | ≤15 | 16-40 | ≥41 | Fisher’s Exact | p-value |
| Euthyroidism | 5 (1.3) | 124 (32.0) | 138 (35.6) | 1.237 | <0.001* |
| Primary hyperthyroidism | 0 (0.0) | 31 (8.0) | 22 (5.7) | ||
| Primary hypothyroidism | 3 (0.8) | 9 (2.3) | 7 (1.8) | ||
| Subclinical hyperthyroidism | 0 (0.0) | 4 (1.0) | 12 (3.1) | ||
| Subclinical hypothyroidism | 7 (1.8) | 7 (1.8) | 10 (2.6) | ||
| Euthyroid sick syndrome | 1 (0.3) | 3 (0.8) | 4 (1.0) | ||
| Euthyroid hyperthyroxinaemia | 0 (0.0) | 0 (0.0) | 1 (0.3) | ||
| Total | 16 (4.1) | 178 (45.9) | 194 (50.0) | ||
Table 2: Spectrum of thyroid diseases in different age groups of patients (n=388).
Table 3 shows the spectrum of TD in different age groups of males only. Apart from ESS found at the extremes of age groups and subclinical hypothyroidism that peaked in the middleage group, there is a direct relationship between age and the occurrence of TD among males. However, the difference is not statistically significant (p=0.09).
| Age groups (years) | |||||
|---|---|---|---|---|---|
| Diagnosis | ≤15 | 16-40 | ≥41 | Fisher’s Exact | p-value |
| Euthyroidism | 1 (1.1) | 23 (25.6) | 40 (44.4) | 33.235 | 0.09 |
| Primary hyperthyroidism | 0 (0.0) | 5 (5.6) | 4 (4.4) | ||
| Primary hypothyroidism | 1 (1.1) | 2 (2.2) | 3 (3.3) | ||
| Subclinical hyperthyroidism | 0 (0.0) | 1 (1.1) | 2 (2.2) | ||
| Subclinical hypothyroidism | 1 (1.1) | 3 (3.3) | 2 (2.2) | ||
| Euthyroid sick syndrome | 1 (1.1) | 0 (0.0) | 1 (1.1) | ||
| Euthyroid hyperthyroxinaemia | - | - | - | ||
| Total | 4 (4.4) | 34 (37.8) | 52 (57.8) | ||
Table 3: Spectrum of thyroid diseases in different age groups of males (n=90).
Table 4 shows the spectrum of TD in different age groups of females. Here, the highest proportion of most TD was found in the 16 - 40 years age group compared with the other age groups. The difference by age group among females was statistically significant (p<0.01).
| Age groups (years) | |||||
|---|---|---|---|---|---|
| Diagnosis | ≤ 15 | 16-40 | ≥ 41 | Fisher’s Exact | p-value |
| Euthyroidism | 4 (1.3) | 101 (33.9) | 98 (32.9) | 13.235 | <0.001* |
| Primary hyperthyroidism | 0 (0.0) | 26 (8.7) | 18 (6.0) | ||
| Primary hypothyroidism | 2 (0.7) | 7 (2.3) | 4 (1.3) | ||
| Subclinical hyperthyroidism | 0 (0.0) | 3 (1.0) | 10 (3.4) | ||
| Subclinical hypothyroidism | 6 (2.0) | 4 (1.3) | 8 (2.7) | ||
| Euthyroid sick syndrome | 0 (0.0) | 3 (1.0) | 3 (1.0) | ||
| Euthyroid hyperthyroxinaemia | 0 (0.0) | 0 (0.0) | 1 (0.3) | ||
| Total | 12 (4.0) | 144 (48.3) | 142 (47.7) | ||
Table 4: Spectrum of thyroid diseases in different age groups of females (n=298).
Discussion
The spectrum of TD among Nigerian patients was evaluated by thyroid function tests at a clinical laboratory. The result of the study was conducted with 69% that indicated euthyroidism with thyroid dysfunction in 31% cases. Thyroid dysfunction included: primary hyperthyroidism (13.7%), subclinical hypothyroidism (6.3%), primary hypothyroidism (4.9%), subclinical hyperthyroidism (4.1%), euthyroid sick syndrome (1.5%) and euthyroid hyperthyroxinaemia (0.3%) in patients in Calabar, Nigeria. Primary hyperthyroidism was the most common form of thyroid dysfunction in women of child-bearing age. The higher frequency of hypothyroidism may be explained by poor iodine nutrition in areas in Nigeria. [15,16]
The high prevalence of TD seen in this study showed that TD is a fairly common endocrine abnormality in contemporary clinical practice. The prevalence found in this study is similar to those reported by previous studies. Baral and co-workers in their study conducted in eastern Nepal, reported a prevalence of approximately 30.0% while Mahato et al. in another region of Nepal found a prevalence of 36.0%. [21,22] Denge et al. in their study reported a prevalence of 31.3%. [23] Amballi et al. in their study carried out in Abakiliki South east, Nigeria obtained a prevalence of 33.9% with individuals between 36 - 45 years of age being mostly affected. [24] The relatively high prevalence of TD in our study may be explained by the geographical location, influence of age, diet and possibly the presence of environmental goitrogens. [15,16]
Furthermore, the high frequency of TD in this study may be related to high prevalence of chronic non-communicable diseases such as obesity, metabolic syndrome, diabetes mellitus (DM), chronic liver disease due non-alcoholic fatty liver disease (NAFLD) which by WHO projection accounted for 24% of deaths in the year 2015. [25] The above-mentioned chronic disease states are pathogenetically-linked and were recently reported to be associated with TD. [26-28] In Nigeria, the current population-based prevalence of DM is not known but a value of approximately 10% was reported based on guestimate. [29] With an estimated population of about 180 million, approximately 18 million Nigerians suffer from diabetes. Both local and foreign studies have reported the common association between diabetes and TD. [30-32]
Recent studies have shown that TD plays a significant role in the etiopathogenesis of NAFLD including non-alcohol steatohepatitis (NASH) and hepatic fibrosis. [33,34] Essentially both subclinical and overt hypothyroidism have been reported to be associated with NAFLD/NASH. [35] A study by Bano and co-workers observed that hypothyroid subjects had higher risk of NAFLD than their euthyroid counterparts. [36] Furthermore, hypothyroidism was found to be associated with increased risk of hepatic fibrosis among patients with early stages of NAFLD. [36] Significantly, obesity, metabolic syndrome, DM and NAFLD – interconnected dysmetabolic states with strong association with underlying insulin resistance – are linked with TD especially subclinical and overt hypothyroidism. [37,38]
In Nigeria, there is paucity of population-based studies on the epidemiology of NAFLD. However, Onyekwere et al. in their study among patients with type 2 diabetes mellitus (T2DM) reported an overall prevalence of NAFLD to be 8.7% among ungrouped subjects with 9.5% in DM patients alone and 4.5% in non-diabetics. [39] Also, Olusanya and co-workers reported prevalence rates of 16.7% and 1.2% among T2DM patients and non-diabetic controls respectively. [40] The relatively high prevalence of TD in type 2 diabetic Nigerians may thus be connected with high prevalence of NAFLD. Even though the influence of high-fat diet on the pathogenesis of NAFLD has been suggested, [41] this has not been ascertained by local Nigerian studies.
Cross river state of Nigeria, with respect to her geographical location spans through regions of environmental and dietary iodine deficiency to those of iodine sufficiency. [15,16] Both iodine deficiency and iodine sufficiency are known to be associated with various patterns of TD. While iodine deficiency is a wellknown cause of TD and goiter, iodine sufficiency has been associated with high incidence of subclinical hypothyroidism. [16-20,42] The northern region of Cross river state belongs to the mountainous belt of east and west African sub-regions that are known for their association with iodine deficiency in soil, food, and water. [15] Both hypothyroidism (due to nutritional iodine deficiency) and toxic nodular or multinodular goiters (with attendant sub-clinical or primary hyperthyroidism) have been reported to occur with greater frequencies in areas of iodine deficiency. [43,44]
The pathophysiology of TD in iodine deficiency states is not far-fetched. Since iodine is essential for biosynthesis of thyroid hormones, insufficient dietary iodine and reduced supply of iodine to the thyroid gland will cause thyroid dyshormogenesis with attendant decreased synthesis, secretion and circulating levels of thyroid hormones. [18,45] This primarily causes hypothyroidism which may be subclinical or overt. Often, iodine deficiency–induced hypothyroidism causes a compensatory increase in synthesis and secretion of TSH which in turn promotes growth and enlargement of thyroid tissue thereby causing goiter (euthyroid or hypothyroid goitre). Most iodine– deficiency goiters are associated with euthyroidism (euthyroid or simple goiters) at the initial stage. Without adequate iodine supplementation, simple goiters may progress to hypothyroid goiters with prevailing subclinicial or primary hypothyroidism. [46] This scenario is common among women of child-bearing age who are known to have increased demand for iodine and also higher prevalence of simple and hypothyroid goiters.
Our study showed that the highest frequency of TD occurred among women between the ages 16 and 40 years, who due to dietary iodine deficiency and increased iodine requirement during pregnancy and lactation, are prone to simple or hypothyroid goiters. Chronicity of iodine deficiency in patients with hypothyroid goiters causes compensatory increase in synthesis and secretion of TSH with increased stimulation of thyroid tissue growth, size, nodullarity, and function thereby causing nodular or multinodular goiters. Multinodular goitres are initially benign but may become toxic with time causing subclinical or overt hyperthyroidism. [44]
Among the spectrum of TD reported in this study, primary hyperthyroidism is the commonest with a frequency of 13.7% with females being more affected (11.3%) than males (2.3%). This particular finding is similar to reports by previous studies. Amballi et al. in a similar study carried out in Sagamu, Ogun state Nigeria, reported prevalence of 25.5% and 8.4% for hyperthyroidism and hypothyroidism respectively. [24] They further observed that primary hyperthyroidism predominated and occurred mostly among patients between 36 - 45 years of age with females being more affected than males. [24] The preponderance of TD among females was also reported by other Nigerian studies. Ogbera and coworkers in their earlier study made similar observation. [1] Again, in a later report by Salami et al, females were noted to be more affected by TD than their male counterparts. [47] Reasons for the higher frequency of TD in females have been suggested and they include: higher prevalence of autoimmune thyroid disease, pregnancy, postpartum thyroid disease, lactation and nutritional factors. [48]
Our study found a total prevalence of subclinical TD to be 10.4% with subclinical hyperthyroidism being 4.1% and subclinical hypothyroidism being 6.3%. These are at variance to findings reported by Eteudo and colleagues in Abakiliki southeast, Nigeria. [49] In their study, the overall prevalence of subclinical hyperthyroidism and sub-clinical hypothyroidism were 14.3% and 4.4% respectively. [49] Similar to our study, they reported higher frequency of TD among females with a femaleto- male ratio of 5.83:1.00.49 The disparity between their study findings and ours may be due to variation in study design and sample size. While their study was cross-sectional in design, this particular study was retrospective.
The higher frequency of subclinical hypothyroidism in our study may be explained in relation to iodine nutrition. Studies have shown that sub-clinical hypothyroidism predominates in areas of iodine sufficiency compared to regions of environmental and dietary iodine deficiency. [7,42] Calabar in Cross river state of Nigeria is located near the sea. Thus, residents are likely to eat more seafoods which are known to be rich in iodine content. In addition, sub-clinical hypothyroidism was observed to occur more in males than in females in our study. This may be related to the relatively high prevalence of the metabolic syndrome as reported by previous studies. [50] Subclinical hypothyroidism has been reported to be a predominant form of TD among patients with the metabolic syndrome. [51] A recent study by Gyawali et al. in India reported an overall prevalence of TD in patient with metabolic syndrome to be 31.8% with sub-clinical hypothyroidism being the predominant form (29.3% of the 31.8%). [52]
Our study reported an overall prevalence of ESS to be 2.1% among the reviewed results. This is relatively low compared to 32.6% and 20.6% found in acute and chronic non-thyroidal illnesses among hospitalized patients. [53] Also a recent study in Nigeria reported a relatively high prevalence of ESS (33.0%) among patients with the metabolic syndrome. [54] The cause of the low prevalence in our study cannot be readily explained. However, the sample size, study design, and the outpatient status of most of the patients involved in our study may be the plausible reasons.
This study has obvious limitations. The first is its retrospective design while the second is the use of purposive sampling technique. In addition, it is an institution-based study carried out within a relatively short time period of 2 years. Large population-based well-designed epidemiological studies with robust sampling technique(s) are needed to establish the epidemiology of TD in the study locality.
Conclusion
Thyroid dysfunction is a fairly common abnormality in our contemporary clinical practice. The spectrum comprises a wide range of disorders including: primary and subclinical hyperthyroidism, primary and subclinical hypothyroidism, euthyroid sick syndrome and euthyroid hyperthyroxinaemia. Primary hyperthyroidism is the most common abnormality and women of child-bearing age were mostly affected. Primaryand subclinical hypothyroidism was more common in males.
Conflict of Interest
All authors disclose that there was no conflict of interest.
REFERENCES
- Ogbera AO, Fasanmade O, Adediran O. Pattern of thyroid disorders in Southwestern region of Nigeria. Ethn Dis 2007; 17: 327-330.
- Rubina M, Syed SRR, Sibga TH, Changa ZK. Spectrum of thyroid diseases, an experience in the tertiary care and teaching hospital. Ann Park Inst Med Sci 2010; 6: 101-106.
- Topliss DJ, Eastman CJ. Diagnosis and management of hyperthyroidism and hypothyroidism. Med J Aust. 2004; 4: 186-193.
- Dufour DR. Laboratory tests of thyroid function: uses and limitations. Endocrinol Metab Clin North Am 2007; 3: 579-594.
- Woeber KA. Subclinical thyroid dysfunction. Arch Inter Med 1997; 157: 1065-1068.
- Biondi B, Cooper DS. The clinical significance of subclinical thyroid dysfunction. Endocr Rev 2008; 29: 76-131.
- Arrigo T, Wasniewska M, Crisafulli G, Lombardo F, Messina MF, Rulli I, et al. Subclinical hypothyroidism: the state of the art. J Endocrinol Invest 2008; 1: 79-84.
- Biondi B, Palmieri EA, Klain M, Schlumberger M, Filetti S, Lombardi G. Subclinical hyperthyroidism: clinical features and treatment options. Eur J Endocrinol 2005; 152: 1-9.
- Helfand M. Screening for subclinical thyroid dysfunction in nonpregnant adults: a summary of the evidence for the US Preventive Services Task Force. Ann Intern Med 2004; 2: 128-141.
- Baloch Z, Carayon P, Conte-Devolox B, Demers LM, Feldt-Rasmussen U, Henry JF, et al. Laboratory medicine practice guidelines: laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid 2003; 1: 3-126.
- Gharib H, Tuttle RM, Baskin HJ, Fish LH, Singer PA, Mc Dermott MT. Subclinical thyroid dysfunction: a joint statement on management from the American Association of Clinical Endocrinologists, the American Thyroid Association and the Endocrine Society. J Clin Endorinol Metab 2005; 90: 581-585.
- McIver B, Gorman CA. Euthyroid sick syndrome: an overview. Thyroid 1997; 1: 125-132.
- Adler SM, Wartofsky L. The non-thyroidal illness syndrome. Endocrinol Metab Clin North Am 2007; 3: 657-672.
- Uy HL, Reasner CA. Elevated thyroxine levels in a euthyroid patient: a search for the cause of euthyroid hyperthyroxinemia. Post grad Med 1994; 5: 195-202.
- Udeozo IOK, Agharanya J. Serum thyrotropin and thyroid hormone levels in normal subjects and those in goitre-endemic areas of Obudu, Nigeria. W. Afr J Med 1986; 5: 209-214.
- Singh A, Etta KM. Thyroid hormone levels in Nigerians living in endemic goiterous and non-goitrous areas. Trop Geogr Med 1978; 30: 235-240.
- Ekpechi OL. Pathogenesis of endemic goitre in Eastern Nigeria Br J Nutr 1967; 21: 537-545.
- Vanderpas J. Nutritional epidemiology and thyroid hormone metabolism. Ann Rev Nutr 2006; 26: 293-322.
- Marwaha RK , Tandon N, Ganie MA, Kanwar R, Garg MK, Singh S. Status of thyroid function in Indian adults: Two decades after universal salt iodization. J Assoc Physicians India 2012; 60: 32-36.
- Ushamenon V, Sundaram KR, Unnikrisgnan AG, Jayakumar RV, Nair V, Kumar H. High prevalence of undetected thyroid disorders in an iodine sufficient adult South Indian population. J Indian Med Assoc 2009; 107: 72-77.
- Baral N, Lamsal M, Koner BC, Koirala S. Thyroid dysfunction in eastern Nepal. Southeast Asian J Trop Med Public Health 2002; 33: 638-641.
- Mahato RV, Nepal AK, Gelal B, Poudel B, Yadav BK, Lamsal M. Spectrum of thyroid dysfunction in patients visiting Kantipur Hospital, Kathmandu, Nepal. Mymensingh Med J 2013; 22: 164-169.
- Denge NS, Thakur AS, Viplav P, Ravikani S, Girishkumar K, Gopikrishna K. Spectrum of thyroid dysfunction in Bastar, Chhattisgarh: A hospital based study. J Cont Med A Dent 2015; 3.
- Amballi AA, Adeleye AO, Oritogun KS, Salu LO. Pattern of thyroid dysfunction in a semi urban Nigerian population through thyroid function tests in a private laboratory, Sagamu, Ogun State, Nigeria. International Journal of Biotechnology 2012; 1: 0146-0149.
- WHO. Facing the facts: The impact of chronic disease in Nigeria.
- Wang C. The relationship between type 2 diabetes Mellitus and related thyroid diseases. J Diabetes Res 2013; 2013: 390534.
- Pearce EN. Thyroid hormone and obesity. Curr. Opin Endocrinol Diab & Obes 2012; 19: 408-413.
- Hage M, Zantout MSM, Azar ST. Thyroid disorders and diabetes mellitus. J Thyroid Res 2011.
- Ogbera AO, Ekpebegh C. Diabetes in Nigeria: The past, present and future. World J Diabetes 2014; 5: 905-911.
- Ghazali SM, Abbiyesuku FM. Thyroid dysfunction in type 2 diabetes seen at the University College Hospital, Ibadan, Nigeria. Nig J Physiol Sci 2010; 25: 173-179.
- Nwokolo HI, Meludu SC, Dioka CE, Onah CE, Ikemefuna FO, Onyema-Ilo OB. The prevalence of thyroid dysfunction and thyroid antibodies among type 2 diabetic patients in Nnewi, South Eastern Nigeria. Journal of Medical Science and Clinical Research 2014; 2: 646-653.
- Snigh G, Gupta V, Sharma AK, Gupta N. Evaluation of thyroid dysfunction among type 2 diabetic Punjab population. Advances in Bioresearch 2011; 2: 03-09.
- Eshraghian A, Jahromi AH. Non-alcoholic fatty liver disease and thyroid dysfunction: a systematic review. World J Gastroenterol 2014; 20: 8102-8109.
- Eshraghian A, Dabbaghmanesh MH, Eshraghian H, Fattahi MR, Omrani GR. Nonalcoholic fatty liver disease in a cluster of Iranian population: thyroid status and metabolic risk factors. Arch Iran Med 2013; 16: 584-589.
- Chung GE, Kim D, Kim W. Non-alcoholic fatty liver disease across the spectrum of hypothyroidism J Hepatol 2012; 57: 150-156.
- Bano A, Chaker L, Plompen EPC, Hofman A, Dehghan A, Franco OH, et al. Thyroid function and the risk of nonalcoholic fatty liver: The Rotterdam Study. J Clin Endocrinol Metab 2016; 101: 3204-3211.
- Martins IJ. Induction of NAFLD with increased risk of obesity and chronic diseases in developed countries. Open J Endocr Metab Dis 2014; 4: 90-110.
- Xu L, Ma H, Miao M, Li Y. Impact of subclinical hypothyroidism on the development of non-alcoholic fatty liver disease: a prospective case-control study. J Hepatol 2012; 57: 1153-1154.
- Onyekwere AC, Ogbera AO, Balogun BO. Non-alcoholic fatty liver disease and the metabolic syndrome in an urban hospital serving an African Community. Ann Hepatol 2011; 10: 119-124.
- Olusanya TO, Lesi OA, Adeyomoye AA, Fasanmade OA. Nonalcoholic fatty liver disease in a Nigerian population with type II diabetes mellitus. Pan Afr Med J 2016; 24: 20.
- Martins IJ. Unhealthy nutrigenomic diets accelerate NAFLD and adiposity in global communities. J Mol Genet Med 2015; 9:1.
- Aminorroaya A, Janghorbani M, Amini M, Hovsepian S, Tabatabaei A, Fallah Z. The prevalence of thyroid dysfunction in an iodine sufficient area in Iran. Arch Iran Med 2009; 12: 262-270.
- Chaudhari RK, Gelal B, Brodie DA, Baral N. Thyroid function and urinary iodine status in primary school age children of the hills and the plains of Eastern Nepal. Indian J Pediatr 2012; 49: 332-333.
- Krohn K, Fuhrer D, Bayer Y, Eszlinger M, Brauer V, Neumann S. et al. Molecular pathogenesis of euthyroid and toxic multinodular goitre. Endocr Rev 2005; 26: 504-524.
- Kirsten D. The thyroid gland: Physiology and pathophysiology. Neonatal Netw 2000; 8: 11-26.
- Triggiani V, Tafaro E, Giagulli VA, Sabba C, Resta F, Licchelli B, et al. Role of iodine, selenium and other micronutrients in thyroid function and disorders. Endocr Metab Immune Disorder Drug Targets 2009; 18: 277-294.
- Salami BA, Odusan O, Ebili HO, Akintola PA. Spectrum and prevalence of thyroid diseases seen at a tertiary health facility in Sagamu, South-West Nigeria. Niger Post grad Med J 2016; 23: 137-140.
- Krassas GE, Poppe K, Glinoer D. Thyroid function and human reproductive health. Endocr Rev 2010; 31: 702-705.
- Eteudo AN, Agu-Eduzor S, Azi SO, Uraku AJ, Igwe CC, Daniel LE. Prevalence of subclinical thyroid disorders and its correlation with age and sex amongst Nigerians residing within Abakiliki metropolis. International Journal of Scientific & Engineering Research 2015; 6: 1522-1530.
- Nwegbu MM, Jaiyesimi OO. Prevalence of metabolic syndrome amongst apparently health of Nigerian adults in a hospital setting. Journal of Medicine and Medical Sciences 2012; 3: 077-082.
- Uzunlulu M, Yorulmaz E, Oguz A. Prevalence of subclinical hypothyroidism in patients with metabolic syndrome. Endocrinol J 2007; 54: 71-77.
- Gyawali P, Tekanche JS, Shrestha RK, Bhattarai P, Khanal K, Risal P, et al. Pattern of thyroid dysfunction in patients with metabolic syndrome and its relationship with components of metabolic syndrome. Diabetes Metab J 2015; 39: 66-73.
- Zargar AH, Garice MA, Masoodi SR, Laway BA, Bashir MI, Wani AI, et al. Prevalence and pattern of sick euthyriod syndrome in acute and chronic non-thyroidal illness: Its relationship with severity and outcome of the disorder. J. Assoc Physicians India 2004; 52: 27-31.
- Udenze I, Nnaji I, Oshodi T. Thyroid function in adult Nigerians with metabolic syndrome. Pan Afr Med J 2014; 18: 352.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.