Survival Status and Treatment Outcome of Multidrug Resistant Tuberculosis (MDR-TB) among Patients Treated in Treatment Initiation Centers (TIC) in South Ethiopia: A Retrospective Cohort Study
2 Department of Public Health, Arba Minch college of Health Sciences, Arba Minch, Ethiopia
Citation: Girum T, et al. Survival Status and Treatment Outcome of Multidrug Resistant Tuberculosis (MDR-TB) among Patients Treated in Treatment Initiation Centers (TIC) in South Ethiopia: A Retrospective Cohort Study. Ann Med Health Sci Res. 2017; 7: 331-336
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Background: The treatment of MDR-TB with second line drug is long, complex and costly, and has a considerable rate of adverse effects. The survival is short and mortality is high, particularly in developing countries. Even though MDR-TB treatment was started in 2013 Survival of MDRTB treatment in peripheral/third generation Treatment Initiation Centers (TIC) was not assessed. Therefore this study tried to assess the survival status and treatment outcome of MDR-TB treatment in the two TIC of SNNPR. Methods: A retrospective cohort study was conducted at Treatment Initiation Centers (TIC) in Yirgalem and Queen Eleni Memorial hospital. Coxproportional hazard regression model was built and variables that had P-value <0.25 in bivariate analysis considered candidates for multivariate analysis to determine independent predictors of mortality by using P-value < 0.05. Results: A total of 154 patients were followed for 186.5 person-year of observation; during the follow up period 13 (8.44%) patients were died making an overall incidence density rate of 7 (95% CI=5.62-8.84) per 100 Person year. Survival at the end of 1st, 2nd, 3rd, and 4th month was 98%, 97%, 95% and 92% respectively. Presence of medical complication [AHR=4.3, 95% CI =2.3-8.71], drug side effect [AHR=2.6, 95% CI =1.7-3.5], seropositivity [AHR=3.15, 95% CI =2.21-6.67], and baseline weight [AHR=0.9, 95% CI =0.82-0.98]. were predicted mortality. Conclusion and recommendations: The incidence of death and treatment outcomes was comparable to other studies. Intervention to further reduce deaths has to focus on patients with comorbidities, HIV, adverse effect and smaller base line body weight.
Keywords
Multi drug resistance; Tuberculosis; Survival status; Treatment outcome; Treatment
Introduction
Multidrug-resistant tuberculosis (MDR-TB) is a type of TB that is resistant to at least the first line anti-TB drugs (Rifampicin and Isoniazid). MDR-TB results from either primary infection or may develop in the course of a patient’s treatment [1]. MDR-TB patients respond poorly to short course chemotherapy and need to be treated intensively for up to 24 months with a regimen based on reserve antituberculosis drugs [2]. The occurrence of MDR- TB is mainly attributable to human error, although genetic factors are believed to contribute to a certain extent [3]. The principal patient-related factor that predicts the occurrence of MDR-TB is non-adherence to treatment [4].
Globally, 5% of TB cases were estimated to have had multidrugresistant TB (MDR-TB) in 2014. Drug resistance surveillance data show that an estimated 480,000 people developed MDRTB and 190,000 people died as a result of MDR-TB in 2014. On average, an estimated 9.7% of people with MDR-TB have XDR-TB. If all notified TB patients (6.3 million) had been tested for drug resistance in 2014, an estimated 300,000 cases of MDRTB would have been detected. In 2014, 123,000 patients with MDR-TB or rifampicin resistant tuberculosis (RRTB) were notified, of whom about 75% lived in the European Region, India, South Africa or China [5].
In 2010, the 27 high MDR-TB burden countries accounted for 85% of all MDR-TB cases. China, and India, was the top two countries accounting 50% MDR-TB cases [6]. The 2010 WHO report showed that the number of MDR-TB cases is rising in Africa [7]. In Africa, where little data is available, an estimated 69,000 cases emerged in 2009, but the vast majority of them went un-diagnosed [8]. Ethiopia is 15th among the 27 MDR-TB high-burden countries, with an estimated 5,200 cases occurring each year [9]. The estimated Multi Drug Resistance cases are 1.6% and 12% among all new and previously treated TB cases respectively. However the extent of drug resistance TB is not well known [10].
The emergence of MDR-TB is a threat for the populations of resource-limited countries. In Ethiopia, the low socioeconomic status of the people, high prevalence of infectious diseases and limited access to well-equipped health care facilities worsens the effect of MDR-TB. Furthermore, poor treatment outcomes, longer treatment time, higher treatment costs, and many more complications make MDR-TB a more complex disease than TB [7,11].
A total of 110,000 patients were enrolled on MDR-TB treatment in 2014, an increase of 14% compared with 2013. Which shows improvement in coverage and access to MDR TB treatment; however the cure rate is low and on the other hand mortality, default and failure are high. Only 50% of the MDR-TB patients in the 2012 cohort of detected cases were successfully treated. 16% died, 24% did not have their treatment outcome documented or interrupted treatment, and in 10% the treatment was failed [5].
The treatment of MDR-TB in Ethiopia started recently in TB specialized hospital and expanded to MDR-TB treatment initiation centers (TIC) in selected hospitals of the regions [10]. However Outcomes of the treatment and associated factors were not described in the peripheral TIC. Therefore, examining a cohort who received a standardized second-line therapy and management of MDR-TB to determine the overall survival rate has a great importance for proper planning and effective implementation. Therefore this study aimed at assessing the Survival Status and treatment outcome of Multidrug resistant Tuberculosis (MDR-TB) Among Patients Treated in Treatment Initiation Centers (TIC) in south Ethiopia.
Materials and Methods
Study design and settings
This institution based retrospective cohort study was carried out in two Treatment Initiation Centers (TIC) of SNNPR; Yirgalem hospital and Queen Eleni memorial hospital. MDRTB treatment was started in St. Piter TB specialized hospital at the end of 2009. Then the service was expanded to selected regional referral hospitals; in 2014 additional 13 MDR TB centers started treatment services, increasing the total number of MDR treatment centers to 32 country-wide, while a total of 332 health institutions were providing follow-up services. In addition there is currently functioning TIC in each selected geographical areas including Arba Minch, Yirgalem and Queen Eleni Mohamed memorial Hospitals in SNNPR. Since 2001, a cumulative total of 1,559 MDR TB patients were enrolled in second line drug (SLD) treatment in Ethiopia along with more than 160 patients in this selected TIC of SNNPR [12].
Study population and sampling technique
All patients with Multidrug resistant Tuberculosis (MDR-TB) who are ever enrolled in the Treatment Initiation Centers (TIC) in South Ethiopia were the source population. Consecutive sampling technique was employed in the two TIC. The number of patients who were ever enrolled in the centers was 160; therefore all patients were included in the study.
Data collection procedure and data quality control
The source of data for the study was individual patient record documents including registers and monitoring cards and patient admission book. Data was collected by using structured checklist that developed from the standard treatment protocol for the management of MDR-TB. The check list sought information on: Patient related data (age, sex, residence), anthropometric measurements (height, weight, BMI), Co-morbidities, types/ category of MDR-TB, medication given, outcome status and dates of admission and events. The data was collected by four health professionals after getting training.
Study variables and data analysis
The Dependent variables is death; the Time variable is Time to occurrence of death measured from admission to date of event and coded as zero (death) and otherwise one; and the independent variables were socio-demographic, anthropometric and clinical presentations. All variables were defined according to the national and WHO MDR-TB management guideline.
Data was cleaned, coded and entered into Epi-info version 7 and exported to SPSS version 21 and STATA version 11, then Exploratory data analysis carried out to check the levels of missing values, presence of influential outliers, multicollinearity, normality and proportionality of hazards over time. Life table was constructed to estimate probabilities of becoming death at different time intervals. Kaplan Meier survival curve together with log rank test was fitted to test for the presence of difference in incidence of death among the groups. Incidence of death with respect to person time at risk was calculated. Variables significant at P<0.25 level in the bivariate analysis were included in the final Cox- regression analysis, to identify independent predictors of mortality.
The final Model was built by forward step wise procedure and compared by likely hood ratio and Harrell’s concordance statistics test. Interactions were tested; Proportionality assumption was tested by global test based on scheonfeld residuals. Goodness of fit of the final model was checked by Nelson Aalen cumulative hazard function against Cox Snell residual. Association was summarized by using adjusted hazard ratio and statistical significances tested at 95% CI.
Ethical statement
The study used the routine existing admission and patient record data of MDR-TB at TIC. There was no direct contact with patients as such, informed consent from the parents was not obtain; however, Ethical approval was obtained from Arba Minch college Health Science ethical review board and support letter was obtained from the college.
Results
Description of admission characteristics
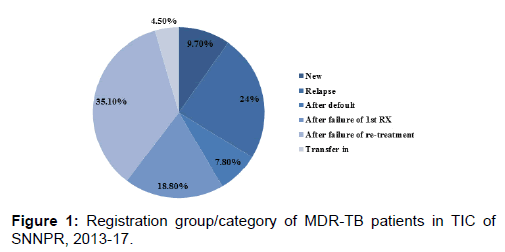
Of the 160 records six have missing data on treatment outcome or date of the treatment outcome. Therefore 154 records with full information on treatment outcome and its date were included in the study. Majorities (59.1%) of the patients enrolled into the study were males and the median age was 27 (IQR=22-35) year, while the median BMI was 15.6 (IQR=11.9-18.7). pulmonary tuberculosis was the dominant one, which is prevalent in 145 (94%) of patients. Also higher proportion of patients (139/154; 90%) had been previously treated for TB, with a median of two episodes. One third (35%) of patients were enrolled after failure of re-treatment, while 29 (18.8%) of them were after default.
However there is no record of history of previous second line drug use [Table 1 and Figure 1].
According to the drug and sensitivity test (DST) result 137(89%) of patients had developed resistance to rifampicin (RIF), 15(9.7%) to isoniazid (INH) and 2(1.3%) to both drugs. In addition two third (69%) of patients had radiographic finding suggestive of Tuberculosis. Opacity, previous TB scar, cavitation/ cavitary lesion, infiltration, infusion and atelectasis were the most prevalent findings [Table 1].
| Variables | Frequency (%) | Median (IQR) | |
|---|---|---|---|
| Age | 27 (22-35) | ||
| Weight | 46.25 (40.6-52.3) | ||
| BMI | 15.6 (11.9-18.7) | ||
| sex | |||
| Male | 91 (59.1) | ||
| Female | 63 (40.9) | ||
| Site | |||
| Pulmonary | 144 (93.5) | ||
| Extra pulm | 5 (3.25) | ||
|  Both | 5 (3.25) | ||
| Have TB treatment history | 139 (90.3) | ||
| Number of episodes (n=139) | |||
| Once | 53 (38%) | ||
| Twice | 82 (59) | ||
| Three times | 4 (3) | ||
| HIV status | |||
| Reactive | 11 (7.1) | ||
| Non-reactive | 143 (92.9) | ||
| Resistance to | |||
| RIF | 137 (89) | ||
| RIF & INH | 15 (9.7) | ||
| More than two | 2 (1.3) | ||
| Substance use | |||
| Yes | 12 (7.8) | ||
| No | 142 (92.2) | ||
| Have comorbidity | 23 (15) | ||
| Developed adverse effect | 106 (69%) | ||
Table 1: Socio-demographic and clinical characteristics of MDR-TB patients in TIC of SNNPR, 2013-2017.
HIV status and other medical conditions
As to the protocol of TB management and TB-HIV care all patients were tested for HIV infection and 11(7.15%) found to have HIV. Of them 9 were diagnosed during their previous TB treatment program and the rest were newly diagnosed. Anti-retroviral therapy (ART) regimen was also initiated for 9 of them according to the National Ethiopian HIV treatment guidelines, while information is missing for the two patients. Significant proportion (15%) of patients also had at least one medical diagnosis other than MDR TB and HIV, Diabetes Mellitus being the most prevalent with (7.2%). In addition to this hypertension, epilepsy, Congestive Heart Failure, Hepatitis and anemia were prevalent in smaller proportion.
Treatment and side effects
Except for some drug changes made after reported side effects the baseline SLD regimen was used for MDR TB treatment with a median of five drugs. Capreomycin (82.3%) was the most common injectable used, followed by amikacin. The median duration of injectable drug use was 8.5 months (IQR 7.2–11.4.0 months) which depends on duration of smear/culture conversion and other factors which delay the conversion like missing of treatments. The most common oral agents used as treatment were ethionamide (99%), levofloxacin (96%) and cycloserine (97%).
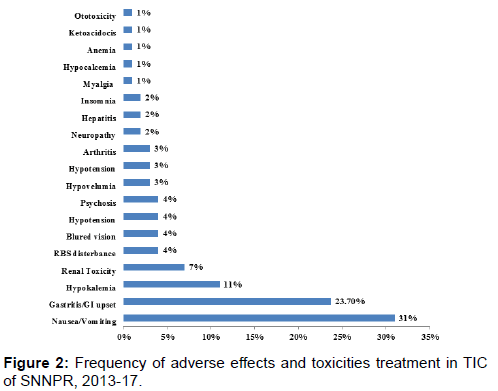
Following initiation of the treatment 106(69%) of patients developed at least one side effect mostly during the first month. Of them the most prevalent side effects were nausea/ vomiting and gastritis/gastric disturbance in 31% and 23.7% of patients respectively. Other side effects; hypokalemia (11%), renal toxicity (7%), blurring of vision (4%), RBS disturbance (4%) and significant proportion of other side effects were also recorded [Figure 2].
Survival status and treatment outcomes
A total of 154 MDR-TB patients were followed for different periods; a minimum of 3 day and a maximum of 777 days with median follow up period of 500(IQR:210-683) days which gives 68,098 person-day of observation. Based on this Incidence rate of death was calculated using Person-day of follow up as a denominator for the entire cohort and for particular groups. Within the follow up period, 13 deaths were recorded. Hence, the overall incidence density rate (IDR) of death in the cohort was 7 (95% CI=5.62-8.84) per 100 Person year and it was significantly different for categories of predictors. The highest incidence rate of Death was observed in the first five months.
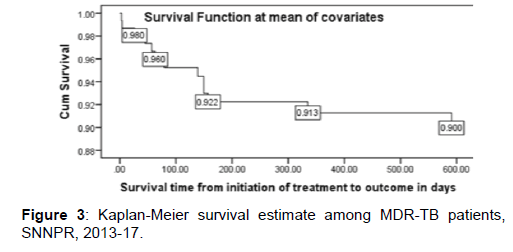
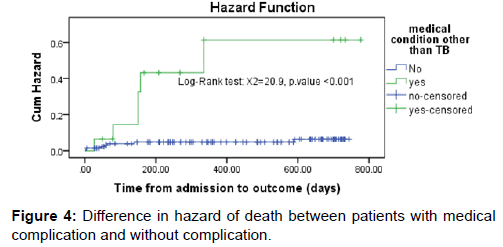
Survival status: The cumulative probability of survival at the end of 1st, 2nd, 3rd, and 4th month was 98%, 97%, 95% and 92% respectively with significant difference between categories of variables, while the overall mean survival time was 23.87(95%CI=22.8-24.93) months. The mean survival time was also significantly different for predictor variables; the median survival time was undetermined. Because the largest observed analysis time was censored, the survivor function does not go to zero; in this case the mean is best estimate of survival time. The difference in hazard of death among variables during the course of intervention period was estimated from Kaplan Meier survival curves with log-rank test and significant difference was observed. The survival of patients with medical complication was significantly shorter [Figures 3 and 4].
Treatment outcomes: During the follow up period 39 (25.3%) patients were cured, 26 (16.9%) were completed their treatment, 13 (8.4%) died during treatment, 20 (13%) defaulted or lost to follow up, 5 (3.2%) of patients required medical transfer and the remaining 51 (33.1%) are on treatment. All deaths were recorded with in the first five months of treatment initiation [Table 2]. Patients were followed for a median of 500 (IQR: 210-683) days on treatment.
| Treatment outcomes | Proportion (%) |
|---|---|
| Cured | 39 (25.3) |
| Completed | 26 (16.9) |
| Died | 13 (8.4) |
| Defaulted | 20 (13) |
| Transferred out | 5 (3.2) |
| On treatment | 51 (33.1) |
Table 2: Treatment outcome of MDR TB patients in SNNPR, 2013-2017.
Factors associated with mortality
Bivariate and Multiple Cox regression: During regression, death was considered as failure and all other outcomes considered as censored. In bivariate analysis, a significant difference was observed between predictors; base line weight, number of previous treatment episode, medical condition or complication other than TB and HIV, HIV sero-positivity and presence of side effect were associated with mortality. By using variables which have p-value of <0.25 in the bivariate analysis Multiple Cox regression was fitted with forward stepwise method. After controlling the effect of other variables presence of medical complication, drug side effect, sero-positivity and baseline weight were predicted mortality from MDR TB while up on treatment [Table 3].
| Predictors | CHR (95%CI) | p-value | AHR (95%CI) | p-value |
|---|---|---|---|---|
| Male Sex | 1.104 (0.30-3.38) | 0.86 | - | - |
| Age | 1.01 (0.97-1.06) | 0.54 | - | - |
| Baseline Weight | 0.94 (0.9-0.99) | 0.02 | 0.9 (0.82-0.98) | 0.01* |
| Extra pulmonary TB | 0.83 (0.43-2.34) | 0.46 | - | - |
| History of previous TB treatment | 2.7 (1.37-4.68) | 0.034 | 1.52 (0.87-5.4) | 0.09 |
| Medical Diagnosis other than TB/co-morbidity | 8.5 (5.7-11.2) | <0.001 | 4.3 (2.3-8.71) | <0.001* |
| Adverse effect | 2.7 (1.3-5.2) | 0.015 | 2.6 (1.7-3.5) | 0.02* |
| HIV sero-positivity | 5.9 (1.8-5.2)) | <0.001 | 3.15 (2.21-6.67) | <0.001* |
| Substance use | 2.1 (0.8-4.9) | 0.17 | - | - |
| Resistance to two or more drugs | 1.9 (0.76-2.67) | 0.092 | 1.24 (0.71-2.16) | 0.12 |
*significant at p-value<0.05
Table 3: Multiple cox regression analysis of factors associated with death.
After controlling the effect of other variables, patients with medical diagnosis other than TB and HIV were 4.3 (95% CI [2.3-8.71], p-value<0.001) times more likely to die than patients without any additional medical diagnosis. The Risk of death was 3.15 (95% CI [2.21-6.67], p-value<0.001) times higher for HIV sero positive patients than HIV sero-negative patients. Similarly the risk of death in patients who developed drug side effect was 2.6 (95% CI [1.7-3.5]. p-value=0.02) times higher than those who didn’t developed side effect. Bigger base line body weight favored the prevention of mortality. On the other hand likely hood of mortality was reduced by 10% as body weight increased by a kilogram [Table 3].
Discussion
This study assessed the survival status and treatment outcome of MDR-TB patients initiated a treatment in the two hospitals of SNNPR, Ethiopia. The number of patients enrolled in the treatment program is increasing from time to time since its establishment as treatment initiation centers partly from increased transmission rate and increased rate of case finding and access to treatment. Within this TIC 154 patients were followed for 186.5 person years, during the follow up 13 patients were died making overall incidence rate of 7 patients per 100 person year of follow-up. Survival at the end of 1st, 2nd, 3rd, and 4th month was 98%, 97%, 95% and 92% respectively and the overall mean survival time was 23.87 (95%CI=22.8-24.93) months. Presence of medical complication, drug side effect, Sero-positivity and smaller baseline weight were reduced the survival and predicted mortality of patients.
The probability of survival at the end of two years in this study which is 88% was higher than a study conducted in St. Piter hospital (Ethiopia), South Africa and Lithuania [13-15]. where a probability of 75-78% was reported. Due to the uses of different technique the mean survival time was different in different research. In our case both the restricted mean (2 year) and extended mean (20 year) was used to describe the mean survival time, however in others the extended mean which is appropriate to estimate when larger proportion of population are censored was used. Incidence of mortality noted above was also by far lower than the report of St. Piter TB referral hospital which was 13.3 patients per 100 person year of observation [13].
The proportion of death in our case is by far lower than many other studies [12,16-20]. However this research is an open cohort in which significant proportion of patients are on treatment in which mortality may be experienced at the later phase of the treatment. A result from a review of 35 articles reported 10.4% mortality, another individual patient review reported 15% death, which is similar to a report of Cox et al. in the same scenario, and two research conducted in Ethiopia also reported a death much higher than our report [13,20].
Overall, 75.3% patients are either completed, cured or alive on treatment throughout the period of follow up with 26 (16.9%) completed, 39(25.3%) patients cured and 51 (33.1%) patients are alive on treatment. This completion rate met the WHO target of 75% [1] and it is higher than the achievement of most MDRTB prevalent countries [5,21]. Also multiples of recent systematic review and meta-analysis reported a lower success rate. Group based meta-analysis Reported pooled treatment success rate of 66.4% [18], also another individual patient based study review reported a success rate of lower than this figure [19]. On the other hand some research from Ethiopia, Kenya, Nigeria and Haiti reported higher success rates [16,20].
The treatment advancement through time, patient condition during admission, program organization, the setting and multitude of other factors may contribute significantly for difference in success rate. Generally in Ethiopia it was noted from WHO reports and previous studies that the success rate was higher [21]. One reason that the success is lower than this findings may be due to higher loss to follow up rate, in which 13% of patients were either lost to follow up or defaulted which markedly reduces the proportion of success full treatments.
Most researchers reported that poor outcome of MDR TB treatment is highly contributed by prevalence of HIV coinfection [13,16,20]. We have discovered the same relation in which the hazard of death in HIV positive individuals was 3 times higher than sero-negatives. This is in line with most recent national and international studies [13,16,20]. This is because during HIV infection the prevalence of co-infection, the probability of drug interaction and side effects and overall compliance will decrease which further increase the hazard of mortality. However, the treatment success can be enhanced through appropriate implementation of ART programs.
The hazard rate of death among patients with co-morbidities or medical complication in this study was 4.3 times higher than their counter parts. Research conducted in Ethiopia also showed the case fatality rate of patients with co-morbidities was higher [13,20]. Similarly the Risk of death for patients with adverse outcomes was higher in another study [22]. Particularly with the prevalence of diabetes mellitus and cor-pulmonale which necessitates intensive care mortality is common due to low compliance, lack of intensive care facility and acute adverse outcomes.
Accordingly, the risk of death among MDR-TB patients who developed drug side effect in the course of treatment was noted higher. It is in line with previous reports [14,23]. in which mortality increases as severe adverse outcomes encountered during treatment. In our study the prevalence of adverse outcome in MDR-TB patient is high with predominance of minor side effects, like gastrointestinal effects which are less likely to be fatal by themself. However it affects the adherence of treatment. In case of severe complication like toxicity, electrolyte disturbance and RBS disturbance patients may experience fatal outcome and also obliged to withhold the treatment which further increase fatality.
Tuberculosis is long been known that it is one of the most chronic infectious disease causing secondary malnutrition. This effect supplemented by gastrointestinal toxicities noted during MDR TB treatment in most patients highly increases the prevalence of malnutrition. In our case the median BMI as a measure of severe acute malnutrition among adults was 15.6 (IQR: 11.9- 18.7) lower than the cut of 16.5. As one component of BMI base line weight was preferred in place of BMI because significant proportion of patients lacks height measurement. We noted that mortality is reduced by 10% as the base line weight increased by a kilogram. This finding is in line with other research [14,15,23]. Similarly Meresa et al. reported BMI had significant association with MDR-TB treatment outcome [20].
On the other hand in contrast to some research [13,16,20]. X-ray findings, history of previous TB treatment and number of drug resistance developed were not significantly associated with treatment outcome. It May be due to epidemiological difference, severity of the cases and adherence to treatment and program organization. In our case most X-ray findings were opacities which are not fatal.
Strength and Limitation of the Study
This research was conducted in second and third generation TIC, most research conducted in Ethiopia is from first generation TIC like St. Peter University of Gondar Referral Hospital. Therefore it may help to measure the case in the newly established TIC. The other strength of this study is that data regarding predictors were collected at admission, before the discharge outcome was known guaranteeing that the measurement of predictor variables was not biased by knowledge of the subjects’ outcomes. Since the outcome is death it is easy to establish temporal relationship with predictor variables which are documented at time of admission.
The findings of this study might suffer from the fact that it is retrospective study and based on records; availability of data for all variables is difficult and those with incomplete information are excluded from analysis. This study did not consider broad ranges of socio-demographic characteristics, biochemical findings and patient management related factors (such as medical supplies and skill of professionals); that might have influenced outcomes. More over the reliability of the recorded data can’t be ascertained and potential bias associated with excluded records and unknown status of absconds were there.
Conclusions and Recommendation
In this study the overall incidence density rate (IDR) of death in the cohort was by far lower than other studies. However, incidence of death was still higher at the first few months of enrolment. The cumulative probability of survival and overall mean survival time was also comparable with other research. Treatment outcomes measured in terms of cure, death, and default rate were comparable to other reports as well. The main predictors of mortality among MDR-TB patients up on treatment were presence of comorbidities, adverse side effects, HIV sero-positivity and smaller baseline weight.
Therefore intervention to further reduce deaths has to focus on patients with comorbidities, severe side effects, HIV infected patients and lower base line body weight. The finding of this research may provide necessary information in areas of improvement; however further research is needed for giving policy level recommendations and addressing missed variables.
Author’s Contribution
All authors have made substantial intellectual contributions to conception, design, and acquisition of data, analysis and interpretation of data to this study. They also have been involved in drafting the manuscript, approved the final manuscript and agreed to be accountable for all aspects of the work.
Acknowledgement
The authors would like to sincerely thank Heads of the Hospitals and data collectors for their greatest contribution. We would also like to acknowledge Arba Minch College of Health Science for facilitating the study.
Conflict of Interest
All authors disclose that there was no conflict of interest.
REFERENCES
- World health organization. Tuberculosis MDR-TB & XDR-TB progress report; 2011.
- WHO. Treatment of tuberculosis: Guidelines For national programs; Geneva; 2003.
- Sharma SK, Mohan A. Multidrug-resistant tuberculosis: a menace that threatens to destabilize tuberculosis control. Chest 2006, 130: 261-272.
- Federal Ministry of Health of Ethiopia (FMOH). Participants’ Manual: national comprehensive tuberculosis, leprosy and TB/HIV training for general health workers. Addis Ababa: FMOH; 2011.
- World Health Organization (WHO). Multidrug-resistant tuberculosis (MDR-TB) 2015 update. Geneva: WHO; 2015.
- World Health Organization (WHO). Multidrug and extensively drug-resistant TB (M/XDR-TB): global report on surveillance and response. Geneva: WHO; 2010.
- World Health Organization (WHO). Drug-resistant tuberculosis now at record levels. 2010.
- World Health Organization (WHO). Global Tuberculosis Control Report; 2010/2011 tuberculosis global facts www.who.int/tb/data; 2010.
- Federal Ministry of Health of Ethiopia (FMOH). Tuberculosis Prevention and Control Programme: special issue for world TB day. 2011. 3:17-37.
- Federal Ministry of Health of Ethiopia (FMOH). Guideline for program and clinical management of drug resistant tuberculosis. Addis Ababa: FMOH; 2009.
- World Health Organization (WHO). Anti-tuberculosis drug resistance in the world: fourth global report. Geneva: WHO; 2008. WHO/HTM/TB/2008.394.
- USAID/Ethiopia. HELP Ethiopia address the low TB performance (HEAL-TB) midterm evaluation. USAID 2014.
- Getachew T, Bayray A, Weldearegay B. Survival and predictors of mortality among patients under multi-drug resistant tuberculosis treatment in Ethiopia: St. Peter’s specialized Tuberculosis hospital, Ethiopia. Int J Pharm Sci Res. 2013; 4: 776-787.
- Balabanova Y, Radiulyte B, Davidaviciene E. Survival of drug resistant tuberculosis patients in Lithuania: retrospective national cohort study. BMJ Open 2011;1: e000351.
- Farley JE, Ram M, Pan W. Outcomes of Multi-Drug Resistant Tuberculosis (MDR-TB) among a Cohort of South African Patients with High HIV Prevalence. PLoS ONE 2011; 6: e20436.
- Macarthur C, Stalz CV, Serena P, Lauren M, Marie M, Oksana O, etal. Treatment outcomes for patients with multidrug-resistant tuberculosis in post-earthquake Port-au-Prince, Haiti. Am. J. Trop. Med. Hyg. 91, 2014, pp. 715–721.
- Orenstein EW, Basu S, Shah NS, Andrews JR, Friedland GH, Moll AP, et al. Treatment outcomes among patients with multidrug-resistant tuberculosis: systematic review and meta-analysis. Lancet Infect Dis 2009, 9: 153-161.
- Amal B, Christopher F, Ejaz Q, Razia F, Katherine F, Ernesto J. A systematic review of the effectiveness of hospitaland ambulatory-based management of multidrug-resistant tuberculosis. Am. J. Trop. Med. Hyg. 2013, pp. 89: 271-280.
- Isaakidis P, Casas EC, Das M, Tseretopoulou X, Ntzani EE, Ford N. Treatment outcomes for HIV and MDR-TB co-infected adults and children: Systematic review and meta-analysis. Int J Tuberc Lung DIS2015; 19:969–978.
- Meressa D, Hurtado RM, Andrews JR. Achieving high treatment success for multi-drug-resistant TB in Africa: Initiation and scale-up of MDR TB care in Ethiopia-an observational cohort study. Thorax Online, 2015. (207374).
- Dennis F, Fuad M, Fraser W, Inés B, Matteo Z, Nguyen L, et al. Multidrug-resistant tuberculosis around the world: what progress has been made? Eur Respir J 2015; 45:150–160.
- Cox HS, Kalon S, Allamuratova S, Sizaire V, Tigay ZN. Multidrug-resistant tuberculosis treatment outcomes in Karakalpakstan, Uzbekistan: Treatment Complexity and XDR-TB among Treatment Failures. PLoS ONE 2007; 2.
- Tang S, Tan S, Yao L, Li F, Li L. Risk factors for poor treatment outcomes in patients with MDR-TB and XDR-TB in China: Retrospective multi-center investigation. PLoS ONE. 2013;8: e82943.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.