Systemic Steroids and Mortality Rate in Severe COVID-19 Infection: Systematic Review and Meta-analysis
2 Medial Intern, Ibn Sina National College for Medical Studies, Jeddah City, Saudi Arabia
3 Pharmacy Intern, University of Tabuk, Tabuk City, Saudi Arabia
4 Medical Intern, Umm Al Qura University, Qunfudhah City, Saudi Arabia
5 General Practitioner, Al Amal Complex for Mental Health, Riyadh City, Saudi Arabia
6 General Practitioner, Ibn Sina National College, Jeddah City, Saudi Arabia
Citation: Kheyam ZA, et al. Systemic Steroids and Mortality Rate in Severe COVID-19 Infection: A Systematic Review and Meta-analysis. Ann Med Health Sci Res. 2021;11: 1208-1211.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Background: Evaluation of posted statistics including on this issue suggests that therapy with corticosteroids in the range of 6 mg of dexamethasone (or equivalent) in keeping with day possibly has an advantageous effect in patients requiring assisted ventilation but there remains considerable doubt in sufferers over the age of 70, in patients with diabetes and patients with milder sickness. Aim: This work aims to determine the safety of systemic steroids and mortality rate in severe COVID-19 patients. Materials and Methods: A systematic search was performed over different medical databases to identify Internal Medicine studies, which studied the outcome of the Steroid group versus the Non-steroid group of COVID-19 patients. Using the meta-analysis process, either with fixed or random-effects models, we conducted a meta-analysis on 28-day mortality rate as a primary outcome, and Serious Adverse Events (SAE rate) as a secondary outcome. Results: Seven studies were identified involving 2583 patients, with 1218 patients in the Steroid group, and 1365 patients in the Non-steroid group. The meta-analysis process revealed a highly significant decrease in the 28-day mortality rate and SAE rate in the Steroid group compared to the Non-steroid group (p < 0.001 respectively). Conclusion: To conclude, steroids have shown their usefulness in critically ill COVID19 patients. However, the efficacy of steroids and steroid dosage remains a matter of argument due to a lack of solid evidence and wide-ranging worries about benefits and harms. Further studies are needed to determine the optimal dosage and duration of steroid therapy which can reduce the mortality rate.
Keywords
Steroids; Mortality; COVID-19
Introduction
Many sufferers had died of severe acute respiratory syndrome (SARS). As defined by way of the centers for disease manage and Prevention (CDC), a suspected SARS patient is a person with a temperature >38°C; scientific findings such as cough, dyspnea, and trouble respiratory, together with the history of journey to an area with the documented local transmission or close contact with a suspected SARS affected person within a couple of days from signs and symptoms onset. [1]
COVID-19 is a new infectious disease inflicting severe respiration failure and death for which gold standard treatment is presently uncertain. Many therapies have been verified to be ineffective, but promising findings associated with corticosteroid remedy had been published. Evaluation of posted statistics including on this issue suggests that therapy with corticosteroids in the range of 6 mg of dexamethasone (or equivalent) in keeping with day possibly has an advantageous effect in patients requiring assisted ventilation but there remains considerable doubt in sufferers over the age of 70, in patients with diabetes and patients with milder sickness. Clinicians must consider the man or woman potential dangers and benefits of corticosteroids in sufferers with COVID-19 in preference to automatically the usage of them till more data is available. [2]
The mortality rate of sufferers with COVID-19 changed to 5% in Wuhan, which becomes near that inside the world (4.2%). Studies try to describe and evaluate the epidemiologic, demographic, scientific, laboratory, and radiological traits in addition to the headaches, remedies, and outcomes of hospitalized sufferers with non-excessive and severe COVID-19. Ability risk elements for severe COVID-19 and factors associated with death in severe cases were analyzed to provide scientific data for relief in severe cases and reduce mortality. [3]
This work aims to determine the safety of systemic steroids and mortality rate in severe COVID-19 patients.
Literature Review
Our review came following the (PRISMA) statement guidelines. [4]
Study eligibility
The included studies should be in English, a journal published article, and a human study describing severely ill COVID-19 patients. The excluded studies were non-English, or animal studies, or describing mild or asymptomatic COVID-19 patients.
Study identification
Searching was conducted over the PubMed, and Google scholar using specific keywords: Steroids, Mortality, COVID-19.
Data extraction and synthesis
RCTs, clinical trials, and comparative studies, which studied the outcome of the Steroid group versus Non-steroid group of COVID-19 patients, will be reviewed.
Outcome measures included a 28-day mortality rate as a primary outcome and on Serious Adverse Events (SAE rate) as a secondary outcome.
Study selection
We found 48 records, 27 excluded based on title and abstract review; 21 articles are searched for eligibility by full-text review; 8 articles cannot be accessed; 2 studies were reviews and case reports; the desired steroid not used in 4 studies leaving 7 studies that met all inclusion criteria.
Statistical analysis
After the pooling of data, Odds ratios were calculated, using MedCalc program (Belgium). Then Q test of heterogeneity, the fixed-effects model or the random-effects models were done.
Results
The included studies were all published in 2020. Regarding patients’ characteristics, the total number of patients in all the included studies was 2583 patients, with 1218 patients in the Steroid group, and 1365 patients in the Non-steroid group and the average age of all patients was (64.5 years) [Table 1]. [5-11]
| N | Author | Number of patients | Age (average years) |
||
|---|---|---|---|---|---|
| Total | Steroid group | Non-steroid group | |||
| 1 | Angus et al. [5] | 197 | 105 | 92 | 60.5 |
| 2 | Bartoletti et al. [6] | 135 | 52 | 83 | 71 |
| 3 | Fernández-Cruz et al. [7] | 463 | 396 | 67 | 67 |
| 4 | Horby et al. [8] | 1007 | 324 | 683 | 59 |
| 5 | Ruiz-Antoran et al. [9] | 506 | 206 | 300 | 70 |
| 6 | Tomazini et al. [10] | 256 | 128 | 128 | 63 |
| 7 | Villar et al. [11] | 19 | 7 | 12 | 61 |
| #Studies arranged alphabetically. | |||||
Table 1: Patients and study characteristics.
A meta-analysis study was done on 7 studies that described and compared the 2 different groups of patients; with an overall number of patients (N=2583) [Table 2]. [5-11]
| N | Author | Primary outcome | Secondary outcome | ||
| 28-day mortality rate | SAE rate | ||||
| Steroid group | Non-steroid group | Steroid group | Non-steroid group | ||
| 1 | Angus et al. [5] | 26 | 29 | 2 | 1 |
| 2 | Bartoletti et al. [6] | 23 | 45 | -- | -- |
| 3 | Fernández-Cruz et al. [7] | 55 | 16 | 18 | 10 |
| 4 | Horby et al. [8] | 95 | 283 | -- | -- |
| 5 | Ruiz-Antoran et al. [9] | 48 | 72 | -- | -- |
| 6 | Tomazini et al. [10] | 69 | 76 | 7 | 15 |
| 7 | Villar et al. [11] | 2 | 2 | 3 | 11 |
Table 2: Summary of outcome measures in all studies.
Each outcome was measured by:
Odds Ratio (OR)
• For 28-day mortality rate.
• For SAE rate.
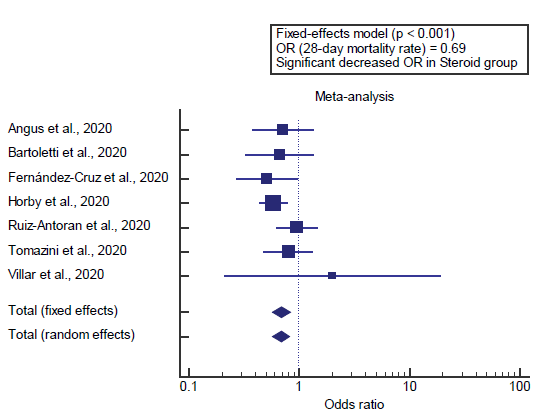
Concerning the primary outcome measure, we found 7 studies reported a 28-day mortality rate with a total number of patients (N=2583). I2 (inconsistency) was 0% with a non-significant Q test for heterogeneity (p>0.05), so fixed-effects model was carried out; with overall OR= 0.69 (95% CI=0.575 to 0.830).
Using the fixed-effects model, the meta-analysis process revealed a highly significant decrease in the 28-day mortality rate in the Steroid group compared to the Non-steroid group (p<0.01) [Figure 1].
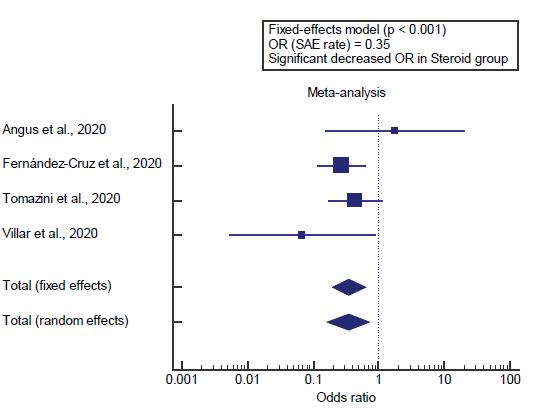
Concerning the secondary outcome measure, we found 4 studies reported SAE rate. I2 (inconsistency) was 23% with a nonsignificant Q test for heterogeneity (p>0.05), so fixed-effects model was carried out; with overall OR= 0.35 (95% CI=0.197 to 0.632).
Using the fixed-effects model, the meta-analysis process revealed a highly significant decrease in the SAE rate in the Steroid group compared to the Non-steroid group (p<0.01) [Figure 2].
Discussion
This work aims to determine the safety of systemic steroids and mortality rate in severe COVID-19 patients.
The included studies were all published in 2020. Regarding patients’ characteristics, the total number of patients in all the included studies was 2583 patients, with 1218 patients in the Steroid group, and 1365 patients in the Non-steroid group and the average age of all patients was (64.5 years).
A meta-analysis study was done on 7 studies that described and compared the 2 different groups of patients; with an overall number of patients (N=2583). Concerning the primary outcome measure, we found 7 studies reported a 28-day mortality rate with a total number of patients (N=2583).
Using the fixed-effects model, the meta-analysis process revealed a highly significant decrease in the 28-day mortality rate in the Steroid group compared to the Non-steroid group (p<0.001), which came in agreement with Westblade et al., [12] Tsang et al., [1] Singh et al., [13] Mikulska et al., [14] Sterne et al., [15] Goyal et al. [16] and Mahase. [17]
Westblade et al. reported that steroid use correlated with chronic lung diseases, the association between inhaled or nasal steroid use and excessive viral load become also discovered restricted to patients with allergies or chronic obstructive pulmonary disorder. In a randomized trial of dexamethasone in hospitalized sufferers with COVID-19, although dexamethasone stepped forward effects of hypoxic sufferers, there was a trend towards accelerated mortality in sufferers who did no longer require oxygen therapy. Steroid therapy has been associated with clearance of SARS-CoV-2. Thus, local use of steroids in the respiratory tract may promote increased SARS-CoV-2 replication. However, the scientific importance of this finding is unsure, specifically given that the use of inhaled or nasal steroids became now not related to extended mortality in our study. [12]
Tsang et al. reported that, predictors of 30-day mortality were PCR positivity (OR 6.4; p=0.038), dyspnea on admission (OR 3.9; p=0.02), presence of important coexisting condition (OR 13.4 p=0.001), total leukocyte count>4 x 109/L (OR, 6.94; p=0.033), and pulsing doses of methylprednisolone (OR 26.0; p=0.001). [1]
Singh et al. reported that, the ventilation free days were more in the dexamethasone group in comparison to controls (12.3 vs. 7.5 days, p<0.01), with a 15.3% reduction of all-cause mortality (21% vs. 36%, p<0.01), 12.5% reduction of ICU mortality (19% vs. 31%, p<0.05) and a 12.5% reduction of hospital mortality (24% vs. 36%, p<0.05). [13]
Mikulska et al. reported that some observations in COVID-19 patients showed that anti-inflammatory treatments could be beneficial. Steroids were associated with decreased mortality. The value of steroids in COVID-19 showed that it was associated with a significant reduction of the risk of death in patients with ARDS (HR=0.38). [14]
Sterne et al. reported that a total of 1703 patients have been covered within the analysis. There had been 222 deaths some of the 678 patients randomized to corticosteroids and 425 deaths amongst the 1025 patients randomized to usual care or placebo (OR, 0.66; P<.001). There has been little inconsistency between the trial effects (I2=15.6%; P=.31) and the OR was 0.70 (P=.053). The fixed-impact precis OR for the association with mortality becomes 0.64 (P<.001) for dexamethasone as compared with usual care or placebo (3 trials, 1282 patients, and 527 deaths). [15]
Goyal et al. reported that exploratory evaluation and visualization indicated age, optimal steroid, severity (measured in P/F) of disorder, and infection repute as potential covariates for survival. Univariate cox regression analysis confirmed the significant effective association of age>60 years and protective impact of most useful steroid {0.38} on death (danger) in seriously sick patients. Multivariate cox regression evaluation after adjusting the impact of age showed a protective impact of optimum steroid on hazard described as death {0.46, LR=17.04}. The concordance turned into 0.7 and version diagnostics fulfilled the assumption standards for the proportional change model. [16]
Mahase reported that, hospitalized with covid-19 who need ventilation, according to preliminary results from the RECOVERY trial. The drug was also found to reduce deaths by one fifth in other hospitalized patients receiving oxygen only, but no benefit was seen among covid-19 patients who did not need respiratory support. [17] Our result came in disagreement with Lee et al. [18]
Lee et al. reported that eight articles (4051 patients) were eligible for inclusion. Among these selected studies, 3416 patients were diagnosed with SARS, 360 patients with MERS, and 275 with COVID-19; 60.3% of patients were administered steroids. The meta-analyses including all studies showed no differences overall in terms of mortality (OR 1.152, p=0.645). [18] Concerning the secondary outcome measure, we found 4 studies that reported SAE rate.
Using the fixed-effects model, the meta-analysis process revealed a highly significant decrease in the SAE rate in the Steroid group compared to the Non-steroid group (p<0.001), which came in agreement with Lee et al., [18] Singh et al. [13] and Sterne et al. [15]
Lee et al. reported that using steroids may be important to prevent sickness progression due to the fact the “cytokine storm” may not be suppressible when the disease is superior. it may be assumed that if the patient does not have severe signs and symptoms, using steroids in low doses may also help to treat coronavirus contamination without complications. [18]
Singh et al. reported that results suggest that early therapy with dexamethasone could change the outcome of ARDS by affecting the systemic immune responses. The effect on the decreased requirement of ventilation is a manifestation of better survival. In ARDS, down regulation of the pulmonary and systemic irritation with long-term dexamethasone ends in a substantial improvement in indices of alveolar-capillary membrane permeability and tissue repair. [18]
Conclusion
To conclude, steroids have shown their usefulness in critically ill COVID-19 patients. However, the efficacy of steroids and steroid dosage remains a matter of argument due to a lack of solid evidence and wide-ranging worries about benefits and harms. Further studies are needed to determine the optimal dosage and duration of steroid therapy which can reduce the mortality rate.
Competing Interests
The authors declare that they have no competing interests. All the listed authors contributed significantly to the conception and design of study, acquisition, analysis, and interpretation of data and drafting of the manuscript, to justify authorship.
REFERENCES
- Tsang OT-Y, Chau T-N, Choi K-W, Tso EY-K, Lim W, Chiu M-C, et al. Coronavirus-positive nasopharyngeal aspirate as predictor for severe acute respiratory syndrome mortality. Emerging Infectious Diseases 2003;9:1381.
- Waterer GW, Rello J. Steroids and COVID-19: we need a precision approach, not one size fits all. Springer; 2020.
- Li X, Xu S, Yu M, Wang K, Tao Y, Zhou Y, et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. Journal of Allergy and Clinical Immunology 2020.
- Liberati A, Altman D, Tetzlaff J, Mulrow C, Gøtzsche P, Ioannidis J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions. Bmj 2009;339.
- Angus DC, Berry S, Lewis RJ, Al-Beidh F, Arabi Y, van Bentum-Puijk W, et al. The REMAP-CAP (Randomized Embedded Multifactorial Adaptive Platform for Community-acquired Pneumonia) Study. Rationale and Design. Annals ATS 2020;17:879–91. https://doi.org/10.1513/AnnalsATS.202003-192SD.
- Bartoletti M, Marconi L, Scudeller L, Pancaldi L, Tedeschi S, Giannella M, et al. Efficacy of corticosteroid treatment for hospitalized patients with severe COVID-19: a multicentre study. Clinical Microbiology and Infection 2020.
- Fernández-Cruz A, Ruiz-Antorán B, Muñoz-Gómez A, Sancho-López A, Mills-Sánchez P, Centeno-Soto GA, et al. A retrospective controlled cohort study of the impact of glucocorticoid treatment in SARS-CoV-2 infection mortality. Antimicrobial Agents and Chemotherapy 2020;64.
- Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, et al. Dexamethasone in hospitalized patients with Covid-19-preliminary report. The New England Journal of Medicine 2020.
- Ruiz-Antoran B, Sancho-Lopez A, Torres F, Moreno-Torres V, Abechuco I de PL de, Lopez PG, et al. COMBINATION OF TOCILIZUMAB AND STEROIDS TO IMPROVE MORTALITY IN PATIENTS WITH SEVERE COVID-19 INFECTION: A SPANISH, MULTICENTER, COHORT STUDY. MedRxiv 2020:2020.09.07.20189357. https://doi.org/10.1101/2020.09.07.20189357.
- Tomazini BM, Maia IS, Cavalcanti AB, Berwanger O, Rosa RG, Veiga VC, et al. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical trial. Jama 2020;324:1307–1316.
- Villar J, Añón JM, Ferrando C, Aguilar G, Muñoz T, Ferreres J, et al. Efficacy of dexamethasone treatment for patients with the acute respiratory distress syndrome caused by COVID-19: study protocol for a randomized controlled superiority trial. Trials 2020;21:1–10.
- Westblade LF, Brar G, Pinheiro LC, Paidoussis D, Rajan M, Martin P, et al. SARS-CoV-2 viral load predicts mortality in patients with and without cancer who are hospitalized with COVID-19. Cancer Cell 2020;38:661–671.
- Singh AK, Majumdar S, Singh R, Misra A. Role of corticosteroid in the management of COVID-19: A systemic review and a Clinician’s perspective. Diabetes & Metabolic Syndrome: Clinical Research & Reviews 2020;14:971–978.
- Mikulska M, Nicolini LA, Signori A, Di Biagio A, Sepulcri C, Russo C, et al. Tocilizumab and steroid treatment in patients with COVID-19 pneumonia. Plos One 2020;15:e0237831.
- Sterne JA, Murthy S, Diaz JV, Slutsky AS, Villar J, Angus DC, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. Jama 2020;324:1330–1341.
- Goyal A, Saigal S, Joshi A, Brahmam D, Niwariya Y, Khurana A, et al. Whether Early Steroid Dose Is Associated with Lower Mortality in COVID-19 Critically Ill Patients-An Exploratory Chart Review. Available at SSRN 3688878 2020.
- Mahase E. Covid-19: Low dose steroid cuts death in ventilated patients by one third, trial finds. British Medical Journal Publishing Group; 2020.
- Lee KH, Yoon S, Jeong GH, Kim JY, Han YJ, Hong SH, et al. Efficacy of corticosteroids in patients with SARS, MERS and COVID-19: a systematic review and meta-analysis. Journal of Clinical Medicine 2020;9:2392.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.