The Effect of Zinc Supplementation on Hair Element Levels in Elite Female Athletes
Citation: Eskici G. The Effect of Zinc Supplementation on Hair Element Levels in Elite Female Athletes. Ann Med Health Sci Res. 2017; 7:150-156
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Background: Zinc is important element for athlete’s performance. Aim: The purpose of this study is to investigate the effect of different doses of zinc supplementation on the hair element levels in elite female athletes. Subjects and Methods: The research was applied on a total number of 20 female athletes playing in volleyball team. The subjects who were randomly allocated into two groups with equal members were administered 220 mg/day (Group 1) and 440 mg/day (Group 2) zinc sulfate. Inductively coupled plasma mass spectrometry (ICP-MS) was used in order to identify magnesium (Mg), calcium (Ca), iron (Fe), copper (Cu), selenium (Se), phosphor (P) and zinc(Zn) levels (μg/g) in the collected hair samples. Results: The analysis of the athletes’ hair samples showed significant difference between the groups only Ca levels (p<0.05, Group 2>Group 1) after 1 and 2 weeks of Zn supplementation. While both doses of zinc supplementation resulted in a significant change in Se levels (p<0.05), the hair Se levels in the last week of administration were significantly lower for both groups compared to the initial levels. Conclusion: While the change in hair element level by weeks was significant in terms of selenium for both groups, which was not significant in terms of Mg, Ca, Fe, Cu, P and Zn levels.
Keywords
Athlete, Hair, Element, Supplementation, Zinc
Introduction
Minerals are essential nutrients which have crucial physical functions in the body. Mineral deficiency affects vital functions, as well as performance with regard to athletes. Magnesium, calcium and phosphorus are some of the major minerals with a daily requirement of >100 mg, while zinc, iron, copper and selenium are some of the minerals classified as trace minerals with a daily requirement of <100 mg, and each mineral has several significant functions in the body [1,2].
Magnesium has major functions in oxygen use, energy metabolism and muscle contraction-relaxation [3-7]. Calcium on the other hand functions in muscle contraction, blood clotting and neural transmission, and helps oxidation of carbohydrates during exercise [8]. Phosphorus participates in energy production during endurance activities, and helps prevention of exhaustion in athletes by showing buffering effect against acid [1]. Iron and copper are responsible for transmission of oxygen available in the structure of hemoglobin and myoglobin [1], and for energy metabolism [9], nervous system [10] and immune function [4,11]. Selenium has antioxidant feature, and protects the cell against free radical damage [1].
Zinc is a biological trace element required for all organisms, and added into the structure of more than 300 enzymes, and required for various metabolic reactions [12-15]. It is an element with a huge role, especially during exercise since it is added to the structure of enzymes that function in carbohydrate, protein and fat metabolism. Owing to its functions such as tissue regeneration, protein synthesis and protection against free radicals, zinc has great importance for recovery after the exercise [1,16]. Depletion of zinc stores negatively affects functioning capacity of musculoskeletal system and athlete performance, leading to tiredness [17].
Zinc and other elements affect absorption of each other when they are taken in excess [2]. Excessive intake of elements such as calcium, phosphorus, iron and copper reduces the absorption of zinc [18], while excessive intake of zinc also reduces absorption of some minerals (iron, copper, calcium, phosphorus and magnesium)[13,19,20].
In biological assessment of the minerals in the body, whole blood, serum and plasma level measurement are frequently used methods [21]. Since minerals in the hair exist in some particular concentrations [22,23] element analysis in the hair, particularly trace element analysis, has appeared as one of the widely used methods in diagnosis of heavy metal toxicity, nutritional status and nutritional deficiency, and some of the diseases in the last three decades [23-26]. Unlike blood, serum and urine, hair is a good marker which gives an idea about the long-term deficiencies [21,26,27].
It is challenging to define reference levels for the trace elements in the hair. Sex, hair color, nutritional habits and some other factors might affect the composition of the hair [24]. Factors such as contamination, hair dyes, shampoos, place of sample collection and hair growth rate might yield misleading results in hair analysis [28]. Besides all these, there are advantages of the hair analysis. It is easily accessible with no pain in sampling, and people are highly likely to give consent to it [29,30].
Although there are studies indicating the existence of an association between zinc in daily nutrition and zinc level in the hair [29] findings are available showing that individuals with low levels of serum zinc have also low levels of hair zinc [31]. The number of studies on the effects of exercise on element metabolism is gradually increasing [1].
The present study investigated whether zinc supplementation of different doses to the elite athletes with an intensive exercise schedule causes any change in the element levels of the hair when it is combined with regular trainings.
There was limited number of studies in the literature regarding the effect of physical exercise on particularly trace element in the hair. Nevertheless, there was no study which demonstrated the effect of zinc supplementation and regular training in elite athletes on hair element level. We believe that this research might be an example for the future studies in this matter.
Materials and Methods
The research was conducted on a total number of 20 female athletes playing in volleyball team in Super League of Sport Club Gazi University whose age, bodyweight, height were 15.1 ± 1.07 years (14-17), 59.9 ± 6.4 kg (50.4-72.2), and 175.3 ± 6.5 cm, respectively. Besides being informed verbally, the athletes (and/or their parents) were provided with a copy of the Helsinki declaration which explained the purpose and performers of the study, and they signed. The study protocol was approved by the Ethical Committee of Higher School of Physical Education and Sports Selcuk University.
The athletes were divided into two groups in equal numbers:
Group 1: The group administered 220 mg/day of zinc sulfate.
Group 2: The group administered 440 mg/day of zinc sulfate.
Hair samples were collected from all athletes, who followed their daily training schedule (6 days/week), 5 times: during the resting period before zinc supplementation (Zn), and once a week during 4 weeks of zinc supplementation, and during resting period following the supplementation. Mg, Ca, Fe, Cu, Se, P and Zn levels (μg/g) in the collected hair samples were determined through ICP-MS device.
The study was conducted during the general preparation period of 8-10 weeks of the athletes (consisting of 70-80% power, and 20-30% technical-tactical exercises).
Daily nutrition of the athletes throughout 5 weeks of study was kept under control with the nutrition program provided by the dietician. This helped them to have energy and nutrients depending on their personal requirement, and particular attention and care was given to prevent insufficient intake of zinc in consumption of nutrients.
The samples were directly stored in zip-mouthed polythene bags, dully labeled with relevant codes. Hair samples with 1 to 2 cm length were clipped with stainless steel scissors from the part closest to the scalp in the suboccipital region. They were first washed with triple distilled water and then with carbon tetrachloride in order to free them from lipids and dust particles. Approximately 0.2 grams of sample was weighted. The sample was taken into the vessel (300°C temperature and 800 psi pressure-resistant vessel) of a close microvawe device. 5 ml of 65% percent nitric acid and 5 ml of deionized water (18MΩ / cm conductivity) was added and were waited during 30 minutes in order for the acid and the water to concentrate with the sample. Vessels were closed and placed the close microvawe device. In the first stage at 190°C and 600 psi, and in the second stage 210°C an 800 psi proccess was enforced. After the end of the program, the vessels were left for a cooldown. When they were at the room temperature, the vessels were opened. Th samples was waited about one hour after 0.5 ml of concentrate hydrogen peroxide was added. The samples put into 25 ml polypropylene volumetric flask. The samples were completed with 25 ml of deionized water (18 MΩ / cm conductivity). They were later analyzed by the device. Samples were read in inductively coupled plasma mass spectrometry (ICP-MS) device (AGILENT 7500 ce, Agilent Technologies, Santa Clara, CA 95051-7201 USA), and Mg, Ca, Fe, Cu, Se, P and Zn levels were determined as μg/g.
The data were evaluated using Statistical Package for the Social Sciences (SPSS 15.0) where the average and frequency distributions were calculated. Further data analysis was conducted through t test, and the difference between groups was determined using Mann Whitney U test. The difference that occurred by weeks was determined through Kruskal-Wallis test. The significance level was considered p<0.05.
Results
The anthropometric measurements of group 1 and group 2 athletes were given in Table 1.
| Anthropometric measurements | Groups | 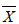 ± S ± S |
|---|---|---|
| Age (year) | Group 1 (n=10) | 14.2 ± 0.42 |
| Group 2 (n=10) | 16.0 ± 0.6 | |
| Weight (kg) | Group 1 (n=10) | 59.7 ± 7.7 |
| Group 2 (n=10) | 60.2 ± 5.1 | |
| Height (cm) | Group 1 (n=10) | 173.6 ± 6.1 |
| Group 2 (n=10) | 177.1 ± 6.6 | |
| Body Fat Percentage (%) | Group 1 (n=10) | 21.3 ± 4.4 |
| Group 2 (n=10) | 17.3 ± 5.4 | |
| Body Fat Mass (kg) | Group 1 (n=10) | 13.0 ± 4.0 |
| Group 2 (n=10) | 10.5 ± 3.9 | |
| Body Fat Free Mass (kg) | Group 1 (n=10) | 46.7 ± 4.4 |
| Group 2 (n=10) | 49.6 ± 4.1 |
Table 1: The anthropometric measurements in Group 1 and Group 2 athletes.
Hair element levels before zinc supplementation, and during 4-week supplementation, and after the end of supplementation
Analyzing hair sample collected from the athletes before zinc supplementation, no significant difference was found between the groups between Mg, Ca, Fe, Cu, Se, P and Zn levels (p>0.05) [Table 2].
| Supplementation time | Groups | Mg (μg/g) |
Ca (μg/g) |
Fe (μg/g) |
Cu (μg/g) |
Se (μg/g) |
P (μg/g) |
Zn (μg/g) |
|---|---|---|---|---|---|---|---|---|
| Before supplementation | Group 1 (n=10) | 288.19± 269.0 | 95.64± 43.60 | 1.45± 0.48 | 33.48± 33.93 | 0.73± 0.39 | 183.66± 62.25 | 155.69± 104.62 |
| Group 2 (n=10) | 327.16± 207.3 | 172.57± 126.50 | 1.56± 0.53 | 24.92± 25.73 | 0.55± 0.14 | 147.94± 51.81 | 177.73± 144.96 | |
| Mann Whitney U | 30.000 | 25.000 | 46.000 | 47.000 | 38.000 | 24.000 | 41.000 | |
| p value | 0.131 | 0.059 | 0.762 | 0.821 | 0.364 | 0.054 | 0.496 | |
| 1weekafter supplementation | Group 1 (n=10) | 269.31± 241.02 | 104.32± 37.06 | 1.85± 0.61 | 27.97± 30.96 | 0.22± 0.13 | 161.05± 64.07 | 130.05± 91.30 |
| Group 2 (n=10) | 295.96± 242.19 | 182.64± 129.39 | 1.99± 0.86 | 20.15± 12.41 | 0.25± 0.13 | 130.59± 48.93 | 214.79± 185.40 | |
| Mann Whitney U | 41.000 | 21.000 | 47.500 | 38.000 | 42.000 | 29.000 | 27.000 | |
| p value | 0.496 | 0.028* | 0.850 | 0.364 | 0.545 | 0.112 | 0.082 | |
| 2 weeks after supplementation | Group 1 (n=10) | 261.50± 231.77 | 109.40± 27.20 | 1.74± 0.51 | 19.91± 18.77 | 0.34± 0.14 | 172.23± 60.02 | 220.04± 184.63 |
| Group 2 (n=10) | 326.86± 233.76 | 224.03± 213.98 | 1.68± 0.54 | 25.14± 16.56 | 0.31± 0.09 | 133.95± 44.61 | 313.29± 290.21 | |
| Mann Whitney U | 38.000 | 17.000 | 45.000 | 30.000 | 46.000 | 27.000 | 39.000 | |
| p value | 0.364 | 0.013* | 0.705 | 0.131 | 0.762 | 0.082 | 0.406 | |
| 3 weeksafter supplementation | Group 1 (n=10) | 217.37± 196.93 | 117.43± 30.18 | 1.84± 0.61 | 18.14± 11.84 | 0.18± 0.06 | 150.32± 43.14 | 188.40± 96.14 |
| Group 2 (n=10) | 324.56± 244.70 | 209.43± 181.51 | 1.88± 0.55 | 20.25± 11.40 | 0.21± 0.13 | 125.41± 28.40 | 268.49± 165.11 | |
| Mann Whitney U | 27.000 | 27.000 | 49.000 | 42.000 | 43.000 | 30.000 | 33.000 | |
| p value | 0.082 | 0.082 | 0.940 | 0.545 | 0.597 | 0.131 | 0.199 | |
| 4 weeksafter supplementation | Group 1 (n=10) | 225.68± 210.79 | 127.77± 31.94 | 1.87± 0.58 | 17.61± 9.22 | 0.28± 0.14 | 153.24± 40.99 | 235.92± 210.87 |
| Group 2(n=10) | 280.31± 215.39 | 199.75± 163.50 | 1.76± 0.50 | 20.40± 8.91 | 0.22± 0.18 | 124.05± 19.66 | 266.88± 187.93 | |
| Mann Whitney U | 39.000 | 33.000 | 46.000 | 33.000 | 36.000 | 25.000 | 43.000 | |
| p value | 0.406 | 0.199 | 0.762 | 0.199 | 0.290 | 0.059 | 0.597 |
Table 2: Changes in hair Mg, Ca, Fe, Cu, Se, P and Zn levels throughout zinc supplementation in athletes in Group 1 and Group 2.
Comparing the hair samples collected 1 and 2 weeks after the beginning of the supplementation, a significant difference was found between Ca levels of the athletes in Group 1 and Group 2, where Ca levels in Group 2 were higher than those of Group 1 (p<0.05) [Table 2]. Hair Ca levels following 4-week zinc supplementation were higher in both groups compared to initial levels.
The result of the analysis of hair samples collected after 3 weeks of supplementation and at the end of the supplementation showed no significant difference between Mg, Ca, Fe, Cu, Se, P and Zn levels between the groups (p>0.05) [Table 2].
Comparison of the changes by weeks in hair element levels in group 1 and group 2 caused by zinc supplementation
Considering the change in the hair element level of the athletes in Group 1 by weeks, zinc supplementation led to a significant change only in selenium level by weeks (p; 0.000<0.05). While hair Se level average was 0.72 ± 0.38 μg/g before the zinc supplementation, it was found to be 0.28 ± 0.14 μg/g at the end of supplementation. Hair selenium level in the last week of zinc supplementation was found to have decreased significantly compared to the level at the beginning of the supplementation [Table 3].
| Elements | Supplementation time | N | 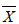 ± S ± S |
Kruskal-Wallis' Z value | p value |
|---|---|---|---|---|---|
| Mg (µg /g) | before supplementation pre- exercise | 10 | 288.18 ± 269.00 | 0.287 | 0.991 |
| 1 week after supplementation | 10 | 269.30 ± 241.02 | |||
| 2 weeks after supplementation | 10 | 261.50 ± 231.77 | |||
| 3 weeks after supplementation | 10 | 217.37 ± 196.93 | |||
| 4 weeksafter supplementation pre- exercise | 10 | 225.68 ± 210.79 | |||
| Ca (µg /g) | before supplementation pre- exercise | 10 | 95.64 ± 43.60 | 5.490 | 0.241 |
| 1 week after supplementation | 10 | 104.32 ± 37.06 | |||
| 2 weeks after supplementation | 10 | 109.40 ± 27.20 | |||
| 3 weeks after supplementation | 10 | 117.43 ± 30.18 | |||
| 4 weeksafter supplementation pre- exercise | 10 | 127.77 ± 31.94 | |||
| Fe (µg /g) | before supplementation pre- exercise | 10 | 1.45 ± 0.48 | 3.497 | 0.478 |
| 1 week after supplementation | 10 | 1.85 ± 0.61 | |||
| 2 weeks after supplementation | 10 | 1.74 ± 0.51 | |||
| 3 weeks after supplementation | 10 | 1.84 ± 0.61 | |||
| 4 weeksafter supplementation pre- exercise | 10 | 1.87 ± 0.58 | |||
| Cu (µg /g) | before supplementation pre- exercise | 10 | 33.47 ± 33.92 | 1.018 | 0.907 |
| 1 week after supplementation | 10 | 27.97 ± 30.96 | |||
| 2 weeks after supplementation | 10 | 19.91 ± 18.77 | |||
| 3 weeks after supplementation | 18.14 ± 11.84 | ||||
| 4 weeksafter supplementation pre- exercise | 10 | 17.61 ± 9.22 | |||
| Se (µg /g) | before supplementation pre- exercise | 10 | 0.72 ± 0.38 | 24.958 | 0.000* |
| 1 week after supplementation | 10 | 0.22 ± 0.13 | |||
| 2 weeks after supplementation | 10 | 0.34 ± 0.14 | |||
| 3 weeks after supplementation | 10 | 0.18 ± 0.06 | |||
| 4 weeksafter supplementation pre- exercise | 10 | 0.28 ± 0.14 | |||
| P (µg /g) | before supplementation pre- exercise | 10 | 183.66 ± 62.25 | 3.488 | 0.480 |
| 1 week after supplementation | 10 | 161.05 ± 64.07 | |||
| 2 weeks after supplementation | 10 | 172.23 ± 60.02 | |||
| 3 weeks after supplementation | 10 | 150.32 ± 43.14 | |||
| 4 weeksafter supplementation pre- exercise | 10 | 153.24 ± 40.99 | |||
| Zn (µg /g) | before supplementation pre- exercise | 10 | 155.68 ± 104.62 | 7.139 | 0.129 |
| 1 week after supplementation | 10 | 130.05 ± 91.30 | |||
| 2 weeks after supplementation | 10 | 220.04 ± 184.63 | |||
| 3 weeks after supplementation | 10 | 188.40 ± 96.14 | |||
| 4 weeksafter supplementation pre- exercise | 10 | 235.92 ± 210.87 |
*p<0.05
Table 3: Changes by weeks in hair element levels in the athletes in Group 1 caused by zinc supplementation.
Considering the change in hair element levels in athletes in Group 2, it was found that zinc supplementation caused a significant change only in selenium level by weeks (p;0.000< 0.001). While hair selenium level average was 0.55 ± 0.14 μg/g before zinc supplementation, it was found to be 0.22 ± 0.18 μg/g at the end. It was determined that hair selenium level in the last week of zinc supplementation decreased significantly compared to the level at the beginning of the supplementation [Table 4].
| Elements | Supplementation time | N | 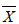 ± S ± S |
Kruskal- Wallis’ Z value | p value |
|---|---|---|---|---|---|
| Mg (µg /g) | before supplementation pre- exercise | 10 | 327.16 ± 207.02 | 1.026 | 0.906 |
| 1 week after supplementation | 10 | 295.96 ± 242.19 | |||
| 2 weeks after supplementation | 10 | 326.86 ± 233.76 | |||
| 3 weeks after supplementation | 10 | 324.56 ± 244.70 | |||
| 4 weeks after supplementation pre- exercise | 10 | 280.31 ± 215.39 | |||
| Ca (µg /g) | before supplementation pre- exercise | 10 | 172.57 ± 126.49 | 1.612 | 0.807 |
| 1 week after supplementation | 10 | 182.64 ± 129.39 | |||
| 2 weeks after supplementation | 10 | 224.03 ± 213.98 | |||
| 3 weeks after supplementation | 10 | 209.43 ± 181.51 | |||
| 4 weeks after supplementation pre- exercise | 10 | 199.75 ± 163.50 | |||
| Fe (µg /g) | before supplementation pre- exercise | 10 | 1.55 ± 0.53 | 4.333 | 0.363 |
| 1 week after supplementation | 10 | 1.99 ± 0.86 | |||
| 2 weeks after supplementation | 10 | 1.68 ± 0.54 | |||
| 3 weeks after supplementation | 10 | 1.88 ± 0.55 | |||
| 4 weeks after supplementation pre- exercise | 10 | 1.76 ± 0.50 | |||
| Cu (µg /g) | before supplementation pre- exercise | 10 | 24.92 ± 25.72 | 1.596 | 0.809 |
| 1 week after supplementation | 10 | 20.15 ± 12.41 | |||
| 2 weeks after supplementation | 10 | 25.14 ± 16.56 | |||
| 3 weeks after supplementation | 20.25 ± 11.40 | ||||
| 4 weeks after supplementation pre- exercise | 10 | 20.40 ± 8.91 | |||
| Se (µg /g) | before supplementation pre- exercise | 10 | 0.55 ± 0.14 | 22.193 | 0.000* |
| 1 week after supplementation | 10 | 0.25 ± 0.13 | |||
| 2 weeks after supplementation | 10 | 0.31 ± 0.09 | |||
| 3 weeks after supplementation | 10 | 0.21 ± 0.13 | |||
| 4 weeks after supplementation pre- exercise | 10 | 0.22 ± 0.18 | |||
| P (µg /g) | before supplementation pre- exercise | 10 | 147.93 ± 51.81 | 1.728 | 0.786 |
| 1 week after supplementation | 10 | 130.59 ± 48.93 | |||
| 2 weeks after supplementation | 10 | 133.95 ± 44.61 | |||
| 3 weeks after supplementation | 10 | 125.41 ± 28.40 | |||
| 4 weeks after supplementation pre- exercise | 10 | 124.05 ± 19.66 | |||
| Zn (µg /g) | before supplementation pre- exercise | 10 | 177.73 ± 144.95 | 5.275 | 0.260 |
| 1 week after supplementation | 10 | 214.79 ± 185.40 | |||
| 2 weeks after supplementation | 10 | 313.29 ± 290.21 | |||
| 3 weeks after supplementation | 10 | 268.49 ± 165.11 | |||
| 4 weeksafter supplementation pre- exercise | 10 | 266.88 ± 187.93 |
*p<0.05
Table 4: Changes by weeks in hair element levels in the athletes in Group 2 caused by zinc supplementation.
Discussion
While zinc supplement consumption leads to increased zinc level in plasma, urine and hair, it is known that excessive intake of zinc negatively affects absorption of iron, copper, calcium and magnesium minerals [20,32-34]. Zinc supplements consumed along with exercises or regular training produce various effects on element metabolism in the body depending on both exercise intensity and duration and dose of zinc supplementation. There are a number of studies and different results in the literature indicating the effects of training on elements in serum, plasma and urine. Although the studies indicating the effect of training along with zinc supplementation on body fluids (serum, plasma, urine) are largely available, there are only limited number of studies on element level in the hair. Although hair analysis is a simple and practical method compared to element analysis in urine and blood, it does not yield results concerning the recent period, but such results are rather indicators of long-term deficiencies or excesses [35,36].
In this present study, no significant difference was found between the groups in terms of Mg, Ca, Fe, Cu, Se, P and Zn levels when hair samples collected from the athletes before the start of zinc supplementation were analyzed. Comparing the hair samples 1 and 2 weeks after the beginning of the supplementation, a significant difference was found regarding only Ca levels between the groups (p<0.05). Ca levels in Group 2 were found higher in both first week (Group 1; 104.32 ± 37.06 μg/g, Group 2; 182.64 ± 129.39 μg/g) and second week (Group 1; 109.40 ± 27.20, Group 2; 224.03 ± 213. 98 μg/g) compared to those in Group 1. Hair calcium levels as a result of fourweek supplementation of zinc were found higher in both groups compared to initial levels. In a study, the hair analysis of the students of Higher School of Physical Education and Sports who had 5 days of mandatory training in a week showed that Mg, Ca and P of female students were higher, while Fe, Na and K of male students were higher [37]. Another study demonstrated that plasma calcium level was increased in athletes who had training along with zinc supplementation [38]. According to the literature information, excessive intake of zinc reduces calcium absorption. Yet, considering the results of that study along with those of the present study, it might be suggested that the increase in calcium level in the hair and plasma could be attributed to the exercise. The fact that calcium level in Group 2, where zinc was administered in a higher dose, was higher compared to Group 1 corroborates this view.
No significant difference was found in terms of hair Mg, Ca, Fe, Cu, Se, P and Zn levels between the groups in the third week and at the end of the supplementation.
Considering the weekly changes of 4-week zinc supplementation along with regular training in elite athletes, only selenium level in both groups had a significant difference (p;0.000<0.05). It was found that selenium levels at the end of zinc supplementation were lower compared to levels at the beginning of the supplementation (for Group 1; 0.72 ± 0.38 μg/g, 0.28 ± 0.14 μg/g, for Group 2; 0.55 ± 0.14 μg/g, 0.22 ± 0.18 μg/g).
There was no significant change by weeks in Mg, Ca, Fe, Cu, Se, P and Zn levels. There are only limited number of studies about the effect of exercise on hair element level. Some elements in plasma and hair of elite female handball players were analyzed on 1st, 7th and 40th day of their pre-summer and summer trainings. At the end of the season, it was found that iron and copper levels in the hair decreased significantly, and there was not significant change in zinc rate [39]. Zaitseva et al. found in their study that copper level in the hair of those who engage in high intensity activity was lower than that of those who engage in low physical activity [40]. It was determined that as the intensity of physical activity increased, the iron and selenium levels in the hair increased as well. The results of the present study show similarity to the findings of the said study regarding the fact that only selenium levels in both groups had significant decrease at the end of the season.
It was demonstrated that acute swimming exercise led to suppression of plasma selenium levels [41] and plasma selenium decreased in the athletes following 7 days of physical activity [42]. The finding that exercise leads to decreased selenium was indicated by Beyleroglu et al. in boxers as well [43]. It was reported that intensive swimming exercise in rats caused a significant suppression of selenium levels, and that such a decrease is a negative factor which reduces performance, and increases production of free radicals [44]. Another study found that urine selenium levels of athletes administered with different doses of zinc (220 mg/day zinc, 440 mg/day zinc sulfate) decreased [45]. The fact that exercise and exercise coupled with zinc supplementation leads to decreased selenium in both serum and urine is in parallel with the decrease marked in hair selenium level as a result of this present study. These results show that exercise and supplementation of zinc within exercise decrease selenium level.
Several studies are available on the effect of exercise and zinc supplementation within exercise on plasma, serum and urine element levels. Besides zinc’s effect on exercise and performance, exercise also has significant effects on zinc and element metabolism [46]. In a study, it was discovered that while short-term high-intensity exercise increases serum Mg concentration, such concentration decreases during long-term endurance exercise [3]. It was found that while serum calcium, zinc and copper levels decreased in wrestlers following acute exercise, phosphorus level increased, and iron and magnesium levels remained unchanged. Following four weeks of regular exercise, the zinc level decreased, and calcium increased. Athletes who were administered 50 mg zinc supplement in addition to training had increased plasma calcium, phosphorus and iron levels, while having decreased magnesium and copper levels [38]. It was demonstrated that oral zinc gluconate supplementation of 22 mg/day to male soccer players for 12 weeks significantly decreased plasma iron [47]. As reported by Cordova et al., rats were provided with 200 ppm oral zinc supplement, and the group of rats which were made to exercise had decreased iron in their serum, while the other group which did not exercise had increased iron in serum [48]. Moreover, it was shown that 12-week zinc gluconate supplementation of 22 mg/day to male children who were playing soccer resulted in copper decrease in plasma [47]. Similarly, 30-day zinc gluconate supplementation of 22 mg/day to bikers significantly decreased plasma copper levels [49]. It was reported that short-term highintensity exercise considerably increased plasma zinc level [50]. Similarly, exercise in marathon runners resulted in increase of plasma zinc level [51]. The increase in plasma levels caused by exercise was also demonstrated by Mundie et al. and Cordova and Navas [52,53].
As clear from the studies mentioned, there are several studies indicating the effects of exercise and zinc supplementation within exercise on serum element metabolism. Yet, no study was encountered regarding the effect of different doses of zinc supplementation to the athletes on element level in hair. Considering literature findings concerning the effect of zinc supplementation on serum, plasma and urine, it was found that element metabolism could be affected by both exercise (intensity, duration, frequency) and zinc supplementation (duration, dose), and that different results might occur. Element analysis in hair reflects more long-term changes compared to analysis in urine and blood. It can be pointed out that only the change of selenium level in this present study as a result of fourweek supplementation of 220 mg and 440 mg of zinc sulfate along with 6 day/week training is in parallel with the decreased selenium levels reported as a result of other studies in literature regarding urine and blood. While hair selenium concentration is considered as a criterion by some countries for assessment of selenium status, the United States does not recognize hair selenium assessment as a valid one due to selenium ingredients of hair products [54]. If hair selenium levels in this study were higher, we could doubt whether this information prejudices our results. On the contrary, we found selenium level lower at the end of four weeks compared to the initial level. We believe that this is an outcome of the combination of training and zinc supplementation.
Conclusion
At the end of the study, it was found that different doses of zinc sulfate supplementation in elite athletes who conducted regular trainings on 6 days a week led to only a significant difference of hair calcium level between the groups in the first and second week. Calcium level in Group 2 was found to be higher than that of Group 1. Hair calcium level in both groups in the fourth week was higher compared to the initial values at the beginning of the supplementation, yet it was not significant. The change in hair element level by weeks in athletes included in Group 1 (220 mg/day zinc sulfate) and Group 2 (440 mg/day zinc sulfate) was found significant with regard to selenium, and selenium levels decreased compared to the beginning of the study. Nevertheless, no significant change occurred by weeks regarding magnesium, calcium, iron, copper, phosphorus and zinc levels.
Acknowledgement
The author would like to offer thanks to Berko Food and Chemical Industry Corporation for providing zinc supplement for the study.
REFERENCES
- Fink HH, Burgoon LA, Mikesky AE. Practical applications in sports nutrition. Canada: Jones and Bartlett Publishers; 2006.
- Insel P, Turner R E, Ross D. Nutrition. 2nd ed. Canada: Jones and Bartlett Publishers; 2004.
- Bohl CH, Volpe SL. Magnesium and exercise. Crit Rev Food Sci Nutr. 2002; 42:533-563.
- Colgan M. Sports nutrition guide minerals, vitamins & antioxidants for athletes. British (Columbia): Apple publishing; 2002.
- Gogus U, Mutluluga dogru gida-spor ve saglik. Ankara: Pelikan yayincilik; 2003.
- Aksoy M. Beslenme Biyokimyasi. Ankara: Hatiboglu yayinevi; 2008.
- Laires MJ, Monteiro C. Exercise, magnesium and immune function. Magnes Res. 2008;21:92-96.
- Ivy J, Portman R. Nutrient timing. The future of sports nutrition. USA: Basic Health Publications; 2004.
- Maughan RJ. Role of micronutrients in sport and physical activity. Br Med Bull. 1999;55:683-690.
- Benardot D. Advanced Sports Nutrition. 2nd ed. Champaign IL: Human Kinetics; 2012.
- Gleeson M, Nieman DC, Pedersen BK. Exercise, nutrition and immune function. J Sports Sci. 2004;22:115-125.
- Cavdar A O. Analysis of zinc (serum, plasma, erythrocyte, and hair zinc) and its relation to nutrition in pregnant Turkish women: A review of cross-sectional and longitudinal studies. J Trace Elem Exp Med. 2000;13:63-71.
- Ulger H, Coskun A. Çinko: Temel fonksiyonlari ve metabolizmasi. Düzce Tip Fak Derg. 2003; 5:38-44.
- Torun MB, Cüce H, Canbilen A. Uzun süreyle oral çinko sülfat kullanilan yasli farelerde timusta meydana gelen histolojik degisikliklerin isik mikroskobu ile arastirilmasi. Genel Tip Derg. 2006;16:93-99.
- Belviranli M, Baltaci AK. The relation between reduced serum melatonin levels and zinc in rats with induced hypothyroidism. Cell Biochem Funct. 2008;26:19-23.
- Krebs NF. Overview of zinc absorption and excretion in the human gastrointestinal tract. J Nutr. 2000;130:1374-1377.
- Pozition of the American Dietetic Association, Dietitians of Canada, and the American College of Sports Medicine: Nutrition and athletic performance. J Am Diet Assoc. 2009;109:509-527.
- Belgemen T, Akar N. Cinkonun yasamsal fonksiyonlari ve Cinko metabolizmasi ile iliskili genler. Ankara Universitesi Tip Fakultesi Mecmuasi. 2004;57:161-166.
- Benito P, Miller D. Iron absorption and bioavailability: An updated review. Nutr Res 1998;18:3581-603.
- Benardot D. Nutrition for serious athletes. 1st ed. Champaign IL: Human Kinetics; 2000.
- Pasha Q, Malik SA, Shaheen N, Shah MH. Investigation of trace metals in the blood plasma and scalp hair of gastrointestinal cancer patients in comoparison with controls. Clinica Chimica Acta. 2010;411:531-539.
- McKenzie JM. Alteration of the zinc and copper concentration of hair. Am J Clin Nutr. 1978;31:470-476.
- Chojnacka K, Gorecka H, Gorecki H. The influence of living habits and family relationships on element concentartions in human hair. Sci Total Environ. 2006;336:612-620.
- Miekeley N, Dias Carneiro MTW, Porto da Silveira CL. How reliable are human hair reference intervals for trace elements? Sci Total Environ. 1998;218:9-17.
- Rodushkin I, Axelsson Md. Application of double focusing sector field ICP-MS for multielemental characterization of human hair and nails. Part I. Analytical methology. Sci Total Environ. 2000;250:83-100.
- Wang CT,Li YJ,Wang FJ,Shi YM,Lee BT. Correlation between the iron, magnesium, potassium, and zinc content in adolescent girl's hair and their academic records. Chang Gung Med J. 2008;31:358-363.
- Ozmen H,Akarsu S,Polat F,Cukurovali A. The levels of calcium and magnesium, and of selected trace elements, in whole blood and scalp hair of children with growth retardation. Iran J Pediatr. 2013;23:125-130.
- Buckley RA, Dreosti IE. Radioisotopic Studies Concerning the Efficacy of Standard Washing Procedures for the Cleansing of hair before zinc analysis. Am J Clin Nutr 1984;40:840-846.
- Erten J, Arcasoy A, Cavdar AO, Cin S. Hair zinc levels in healthy and malnourished children. Am J Clin Nutr. 1978;31:1172-1174.
- Pena-Fernandez A,Gonzalez-Munoz MJ,Lobo-Bedmar MC. Reference values of trace elements in the hair of a sample group of spanish children (aged 6-9 years)-Are urban top soils a source of contamination? Environ Toxicol Pharmacol. 2014;38: 141-152.
- Strain WH, Steadman LT, Lankau CA, Berliner WP, Pories WJ. Analysisi of zinc levels in hair fort he diagnosis of zinc deficiency in man. J Lab Clin Med. 1966;68:244-249.
- Irving JA, Mattman A, Lockitch G, Farrell K, Wadsworth LD. Element of caution: a case of reversible cytopenias associated with excessive zinc supplementation. CMAJ. 2003;169:129-131.
- Kavas A. Saglikli yasam icin dogru beslenme. 3. basim. Istanbul: Mart matbaacilik; 2003.
- Maret W, Sandstead HH. Zinc requirements and the risks and benefits of zinc supplementation. J Trace elem Med Biol. 2006;20:3-18.
- Bass DA, Hickock D, Quig D, Urek K. Trace element analysis in hair: factors determining accuracy, precision, and reability. Altern Med Rev.2001;6:472-481.
- Rodrigues JL,Batista BL,Nunes JA,Passos CJ,Barbosa F JR. Evaluationof the use of human hair forbio monitoring the deficiency of essential and exposure totoxic elements. Sci Total Environ.2008;405:370-376.
- Lubkowska A. Concentration of selected elements in hair of healthy individuals with increased physical activity. Trace Elem Electrolytes. 2009;26:145-149.
- Karakukcu C,Polat Y,Torun YA,Pac AK. The effects of acute and regular exercise on calcium, phosphorus andtrace elementsin young amateur boxers.Clin Lab.2013;59:557-562.
- Zhang Y. Dynamic Study on the Content of Both Hair and Blood Plasma Iron, Zinc, Copper and Manganese in Elite Female Handball Athletes During High Intensity Summer Training. 2003;04.
- Zaitseva IP,Skalny AA,Tinkov AA,Berezkina ES,Grabeklis AR,Skalny AV.The influence of physical activity on hair toxic and essential trace element content in male and female students. Biol Trace Elem Res. 2015;163:58-66.
- Baltaci AK, Uzun A, Kilic M, Mogulkoc R. Effects of acute swimming exercise on some elements in rats. Biol Trace Elem Res. 2009;127:148-153.
- Margaritis I, Rousseau AS, Hininger I, Palazzetti S, Arnaud J, Roussel AM. Increase in selenium requirements with physical activity loads in well-trained athletes is not linear. Biofactors. 2005;23:45-55.
- Beyleroglu M, Savas S, Hazar M. Effect of technical sportive loading upon themalondialdehyde (MDA) and trace metals levels of boxers. Ovidius University Annals. 2010;10:8-13.
- Jana K, Samanta PK, Manna I, Ghosh P, Singh N, Khetan RP, et al. Protective effect of sodium selenite and zinc sulfate on intensive swimming-induced testicular gamatogenic and steroidogenic disorders in mature male rats. Appl Physiol Nutr Metab. 2008;33:903-914.
- Eskici G, Gunay M, Baltaci AK, Mogulkoc R. The effect of zinc supplementation on the urinary excretion of elements in female athletes. Pak J Pharm Sci. 2016; 29:125-129.
- Baltaci AK. Cinko ve egzersiz. Genel Tip Derg. 2001;11:129-133.
- Oliveira KJF, Donangelo CM, Oliveira AV, Silveira CLP, Koury JC. Effect of zinc supplementation on the antioxidant, copper, and iron status of physically active adolescents. Cell Biochem Funct. 2009;27:162-166.
- Cordova A, Navas FJ, Escanero JF. The effect of exercise and zinc supplement on the hematological parameters in rats. Biol Trace Elem Res. 1993;39:13-20.
- Marques LF, Donangelo CM, Franco JG, Pires L, Luna AS, Casimiro-Lopes G, Lisboa PC, Koury JC. Plasma Zinc, Copper, and Serum Thyroid Hormones and Insulin Levels After Zinc Supplementation Followed by Placebo in Competitive Athletes. Biol Trace Elem Res. 2011;142:415-423.
- Ohno H, Yamashita K, Doi R, Yamamura K, Kondo T, Taniguchi N. Exercise- induced changes in blood zinc and related proteins in humans. J Appl Physiol. 1985;58:1453-1458.
- Marrella M, Guerrini F, Solero PL, Tregnaghi PL, Schena F, Velo GP. Blood copper and zinc changes in runners after a marathon. J Trace Elem Electrolytes Health Dis. 1993;7:248-250.
- Mundie TG, Hare B. Effects of resistance exercise on plasma, erythrocyte, and urine Zn. Biol Trace Elem Res. 2001;79:23-28.
- Cordova A, Navas FJ. Effect of training on zinc metabolism: changes in serum and sweat zinc concentrations in sportsmen. Ann Nutr Metab. 1998;42:274-282.
- King JC. Specific nutrient requirements trace elements. In: Gershwin ME, German JB, Keen CL, editors. Nutrition and immunology and practise. Totowa (New Jersey): Humana pres. 2000; 65-72.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.