Understanding Neuromuscular Monitoring
2 Department of Anesthesiology and Intensive Care, Collegium Medicum University of Warmia and Mazury in Olsztyn, Olsztyn, Poland
3 Department of Anesthesiology and Intensive Care, Pro-Medica Hospital, Institution of ELK, Olsztyn, Poland
4 Department of Anesthesiology and Intensive Care, Non Public Health Care Institution-Health, Olsztyn, Poland
Received: 17-May-2022, Manuscript No. AMHSR-22-63991; Editor assigned: 20-May-2022, Pre QC No. AMHSR-22-63991(PQ); Reviewed: 06-Jun-2022 QC No. AMHSR-22-63991; Revised: 18-Jul-2022, Manuscript No. AMHSR-22-63991(R); Published: 25-Sep-2022
Citation: Radkowski P, et al. Understanding Neuromuscular Monitoring. Ann Med Health Sci Res. 2022;12:1-6.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Introduction: Neuromuscular blocking agents are an important component of anesthesia. The effect of muscle relaxants is possible to evaluate by using instrumental objective neuromuscular monitoring. It cannot be determined by clinical tests. The procedure that allows us to measure the muscular response to electrical stimulation of the compatible motor nerve is called relaxometry. Despite anesthesia societies’ recommendations, objective neuromonitoring is still not routinely used in clinical practice.
https://sporbahisleri.blogaaja.fi http://sporbahisleri.parsiblog.com https://spor-bahisleri.jimdosite.com https://sporbahisleri.edublogs.org https://sporbahisleri.websites.co.in https://sporbahisleri.podia.com https://sporbahisleri7.wordpress.com https://sporbahisleri.jigsy.com https://niwn-chroiaty-mcieung.yolasite.com https://spor-bahisleri.mywebselfsite.net https://sporbahisleri.mystrikingly.com https://sporbahisleri.splashthat.com https://sporbahisleri1.webnode.com.tr https://sporbahisleri.odoo.com http://sporbahisleri.creatorlink.net http://www.geocities.ws/sporbahisleri/ https://spor-s-site.thinkific.com https://artistecard.com/sporbahisleri https://sporbahisleri.estranky.cz https://spor-bahisleri.mozellosite.com https://651be6b563e56.site123.me https://betsitesiinceleme.blogspot.com https://sporbahisleri.hashnode.dev https://sporbahislerim.wixsite.com/spor-bahisleri https://sporbahislerix.weebly.com https://sites.google.com/view/betsiteleri https://codepen.io/sporbahisleri https://sporbahisleri.bcz.com https://www.smore.com/6rsb9
Aim: The aim of this paper is to present the basic principles of neurostimulation, patterns of nerve stimulation and equipment used for neuromuscular monitoring.
Result: The adductor pollicis muscle is routinely used to monitor neuromuscular transmission (gold standard), nevertheless it cannot be regarded as representative for some muscle groups such as the larynx, diaphragm, and eye muscles which are more resistant against no depolarizing muscle relaxants.
Discussion: The Train of Four stimulation (TOF) predicts the optimal time for intubation, maintains the desired depth of the intraoperative blockade and the correct estuation time. Monitoring of deep muscular blockade is only possible with the Post Tetanic Count (PTC). Acceleromyography is the most popular method of neuromuscular monitoring.
Conclusions: Because there is an individual variability in the action of muscle relaxants, every surgery the requires the administration of these drugs must be objectively monitored. Exclusion of residual neuromuscular block is possible only when the TOF ratio ≥ 0.9 in electromyography, compressomyography and kinemyography or=1,0 in acceleromyography.
Keywords
Electromyography; Acceleromyography, Kinemyography
Introduction
Neuromuscular blocking agents are an important component of modern anesthesia. They facilitate tracheal intubation and improve operative conditions in abdominal and thoracic surgery. In emergency care, muscle relaxation is used during mechanical ventilation in the prone position to minimize the patient’s breathing and to improve oxygenation. The effect of delivery of muscle relaxants is only evaluable with objective instrumental neuromuscular monitoring. It cannot be determined by clinical tests (muscle tone, head lift, eyeopening, tongue protrusion, handgrip, tidal volume, peak inspiratory pressure). Neuromuscular residual blocks are perceived not only as uncomfortable, but also as a lifethreatening condition. Aspiration and obstruction of the upper respiratory tract occur quite often, which can lead to serious pulmonary complications. The procedure that allows us to measure the muscular response to electrical stimulation of the compatible motor nerve is called relaxometry [1]. The first reports of guidelines for dealing with patients treated with non-depolarizing muscle relaxants were published in 2009 at the initiative of the working party of post anesthesia care [2]. In 2018, a group of experts set basic guidelines for dealing with patients after administration of muscle relaxants [3]. They postulate that any patient after administration of a non-depolarizing relaxant should be subjected to objective neuromonitoring, as neither subjective methods or clinical trials are sufficiently sensitive to the residual neuromuscular blockade detection and should not be practiced. Important anesthesia societies: the European Society of Anesthesiology (ESA) and the American Society of Anesthesiology (ASA) still have not published guidelines, but in some countries, Anesthesiology Associations have published detailed ones, e.g., in the UK and Ireland (2015), the need for neuromonitoring has been highlighted in all patients who have been administered muscle relaxants from the time of induction to the complete return of neuromuscular transmission (TOF ratio>0.9) and the patient’s consciousness[4]. In Poland, according to the "Regulation of the Minister of Health of December 2016 on the organizational standards of healthcare in the field of anesthesiology and intensive care”, there should be a device for neuromuscular transmission monitoring designated for each patient station [5]. Despite anesthesia societies’ recommendations, objective neuromonitoring is still not routinely used in clinical practice.
Aim
The aim of this paper is to present the basic principles of neurostimulation, patterns of nerve stimulation and equipment used for neuromuscular monitoring.
Literature Review
Complete recovery of neuromuscular function (TOF grater to 0.9) should be present at the time of tracheal extubation. In comparison to other groups of drugs, response to clinical doses of muscle relaxants is individual for each patient and is difficult to predict. The application of qualitative monitoring is associated with an increased risk of a residual neuromuscular block [6].
Neurostimulation
For the initiation of an action potential, a stimulus of sufficient intensity (above the threshold value) is required to depolarize the neuron. After stimulation, a single nerve fiber will always give a maximum response and action potential of the same amplitude. Even if the intensity of the stimulus is increased, the peak of the action potential always remains the same. The nerve fiber gives a maximum response or none at all. This is called the "all or none" principle. In neurostimulation we use a stimulus with certain parameters. It should be a supramaximal stimulus with electric current 60-80 mA to stimulate all muscle fibers. It should also be monophasic, rectangular; with duration of 0.2 ms. longer duration can stimulate the muscle directly.
Stimulation electrodes
The negative electrode should be placed directly over the nerve. The positive electrode should be placed proximal to the negative electrode in order to avoid depolarizing a different nerve. Such conditions ensure optimal nerve stimulation. The exact location of the electrodes should be determined to avoid direct stimulation of the muscle. This situation will result in false measurement. There should be a gap between the electrodes placed on a patient's skin [7].
Stimulation points
Neuromuscular block can be monitored by stimulating different nerves and measuring the response of the muscles innervated by the relevant nerve. The relaxometry of the ulnar nerve at the adductor pollicis is the gold standard in anesthesiology. There are also alternative muscles such as: flexor hallucis brevis, orbicularis oculi or corrugator super cilia [8].
N. ulnaris and M. adductor pollicis
The ulnar nerve is stimulated for neuromuscular monitoring and in effect the abduction of the thumb is assessed as the stimulatory response. The ulnar nerve is easy to locate at the distal part of the ulna and has no risk of direct muscle stimulation.
N. tibialis posterior and M. flexor hallucis brevis
The flexor hallucis brevis muscle can be used for neuromuscular monitoring on the lower limb when the adductor pollicis is not accessible. Previous studies have compared posterior tibial nerve and ulnar nerve stimulation and have found more rapid recovery time of the TOF response at the posterior tibial nerve [9-12]. Monitoring of the foot may result in an overstimulation of the neuromuscular block. In the newest publication from 2020, which compares the flexor hallucis brevis and adductor pollicis using acceleromyography, authors concluded, that flexor hallucis brevis can be a more useful alternative for NMB monitoring in cases in which the adductor pollicis is not accessible, but should be used only with available pharmacological reversal (Table 1) [13].
| Table 1: Sensitivity of different muscle groups to neuromuscular conduction blockers blocking agents. |
|---|
| Pharyngeal muscles |
| Masseter muscle |
| Genioglossus muscles |
| Adductor pollicis muscle |
| Abdominal muscles |
| Orbicularis occuli muscle |
| Vocal cords muscles |
| Corrugator supercilli muscle |
| Diaphragm |
N. facialis and M. orbicularis oculi/M. corrugator supercoil
Stimulation of the facial nerve and monitoring of the muscular response around the eye (the orbicularis oculi muscle typical muscle response: eyelid closes and the corrugator supercoil muscle (typical muscle response brow wrinkles) are performed during general anesthesia. The orbicularis oculi muscle and the corrugator supercoil muscle react to NMBA with varying sensitivities. The neuromuscular block at the orbicularis oculi muscle is similar to that of the adductor pollicis; the corrugator supercilii has similar sensitivity to that of the laryngeal adductor muscles or diaphragm. In case of the facial nerve, we can expect false-positive responses because the associated muscle group may be easily stimulated directly, potentially causing an overdose of muscle relaxants [14].
Anesthesia-relevant muscle groups
Different muscles have individual onset, recovery times and sensitivities to muscle relaxants. The results obtained from one muscle cannot be applied to other muscles. The diaphragm and other respiratory muscles are more resistant against non-depolarizing muscle relaxants than the adductor pollicis muscle. The diaphragm requires about double the amount of muscle relaxant as the adductor pollicis muscle for the same degree of block. However, the onset and recovery times are relatively rapid as a result of high regional blood flow. The muscles of the larynx and face are less resistant. Adequate intubating conditions can be well predicted if the corrugator supercilious is monitored because this muscle reflects the extent of NMBA of the laryngeal muscles better than that of the adductor pollicis. The peripheral muscles of the limb, abdominal muscles, the masseter, and the upper airway muscles are more sensitive. However, peripheral muscles of the limb require the longest time to recover. After administration of NMBA the adductor pollicis muscle recovers earlier than the throat, masseter and genioglossus muscles, that’s why there is a danger of missing the residual block of those muscles while the adductor pollicis is monitored. The precise source of these differences is unknown. Possible causes may be the difference in the margin of safety of the neuromuscular junction of different muscle groups, fiber composition, and innervation to blood flow ratio and muscle temperature. It is probable that due to higher blood flow in central muscles, faster onset and recovery time is observed. In peripheral muscles, lower blood flow results in shorter onset and recovery time. Whereas, neuromuscular resistance of laryngeal muscles and diaphragm can be the result of higher ACh vesicles release and higher Ach receptors expression [14].
Discussion
Patterns of nerve stimulation
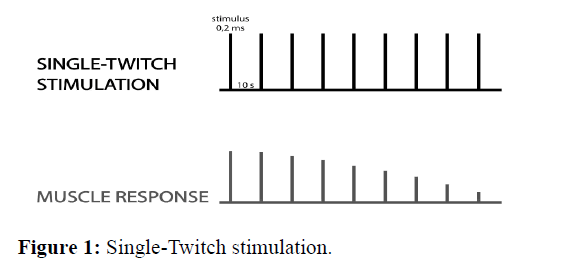
Single-Twitch stimulation this is the easiest way to stimulate the nerve. It consists of applying individual stimuli with an interval of more than 10 s (between 0,1 Hz and 1,0 Hz) and comparing the strength of muscle contraction to the control value before the administration of the muscle relaxant. The amplitude of muscle contraction decreases when about 75-80% of the receptors are blocked, and it disappears when 90-95% of the receptors are blocked, so this method is not commonly used (Figure 1)[14].
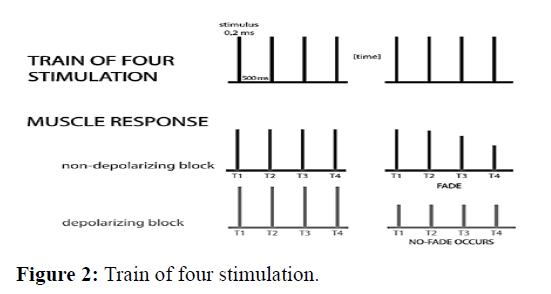
Train of four stimulation this is the most widely used mode of nerve stimulation. Four supramaximal stimuli of 2 Hz (four stimuli every 0.5 s) are applied over 2 second intervals, which are repeated every 10 s. The response is observed as TOF count (0,1,2,3 or 4) or TOF ratio (from 0 to 1.0 or 100%). TOF ratio (TOF %) is the ratio of the fourth muscle response to the first one. TOF% indicates a fade in the nondepolarizing block. Clinically, the rule is: if only 1-2 contractions are obtained, this corresponds to a blockage of 90-95%. This method is only useful for the no depolarizing blockade because after administration of a depolarizing drug, the amplitude of all 4 contractions decreases equally and the 4 to 1 contraction ratio is always 1.0, no fade occurs. During a partial non-depolarizing block, the ratio decreases and is inversely proportional to the degree of blockade (Figure 2) [14].
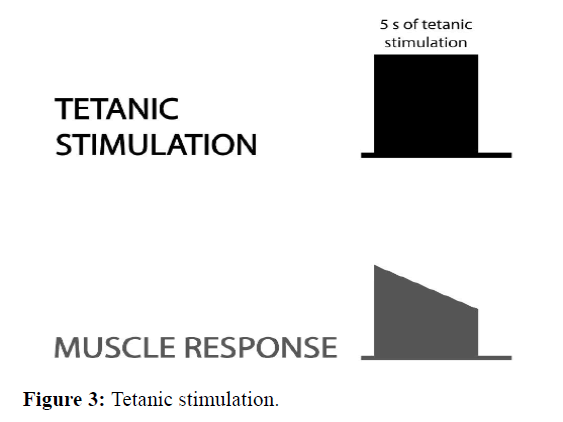
Tetanic stimulation delivering high-frequency stimulation 50 Hz for 5s; the induced muscle contractions merge. During normal neuromuscular transmission and depolarizing block, the muscle response is sustained. In case of no depolarizing block, a fade occurs (the response is not sustained). This method is used in practice only with post-tetanic count (Figure 3) [14].
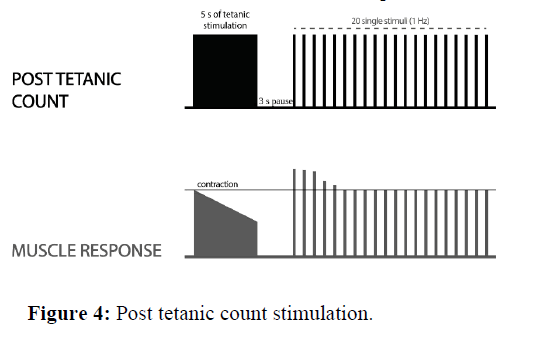
Post Tetanic Count stimulation (PTC) when no responses are detected to TOF stimulation (in deep neuromuscular block), the PTC is the only way to measure the neuromuscular block. The tetanic stimulation (50 Hz) is generated for 5 s. After that, the single-twitch stimulation is given (1 Hz, starting 3 seconds after the end of tetanic stimulation) and the post tetanic response is observed. As the deep block weakens, more and more responses to post-tetanic twitch stimulation appear. The number of detected responses using the PTC module returns sooner than normal TOF responses (Figure 4).
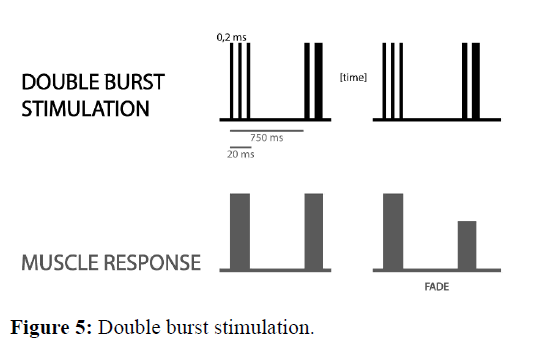
Double Burst Stimulation (DBS) it consists of two short bursts of 50 Hz tetanic stimulation within 750 msec. Most commonly, there are 3 impulses in each of the tetanic bursts. This pattern of stimulation was developed to allow manual detection of small amounts of residual blockade in clinical practice. In the partly paralyzed muscle, the second response is weaker than the first one. After stimulation, the ratio between the second and first contraction is assessed. However, it allows to detect the difference between two muscle contractions only to TOFR=0.6. Due to this fact it is not possible to detect residual neuromuscular blockade using only DBS (Figure 5) [14].
Methods of neuromuscular monitoringClinical tests of the muscle function and of the evaluation of respiratory parameters. For decades, the degree of the neuromuscular blockade has been assessed on the basis of clinical tests. Unfortunately, this is still the case today, even though this procedure is unreliable and can cause lifethreatening conditions. There are several different clinical tests (Table 2) that can be used to assess the degree of muscle relaxation of a patient.
| Table 2: Clinical tests for assessing neuromuscular recovery. | |
|---|---|
| Non-Instrumental (subjective) clinical tests | |
| Unreliable | Less unreliable |
| Hand raising | Hand grip>5 s |
| Eyes opening | Head lift>5 s |
| Sticking out the tongue | Leg lift>5 s |
| Normal tidal volume | Tongue depressor test for 5s |
| Maximal inspiratory pressure<40-50 cm H2O | Maximal inspiratory pressure>40-50 cm H2O |
The 5-s head lift is not a reliable test, because it can be performed at TOFR 0.5 in more than 70% of patients [15].
Extubating patients in this stage of neuromuscular recovery may result with difficulties breathing and other complications. In Camu publication from 2006 it is stated that that none of the clinical tests have a sensitivity of >0.35 or a positive value of >0.52 [16]. One of the difficulties in conducting clinical tests is that they require good patient cooperation and patient responsiveness, which is often difficult to achieve.
Subjectively (Qualitative) evaluation
Qualitative monitoring uses peripheral nerve stimulators and are able to assess visual or tactile response of the stimulated muscle. A standard peripheral nerve stimulator can measure several patterns of nerve stimulation such as TOF, DBS, tetanic and PTC. Quantitative monitoring is more reliable than clinical tests, but does not eliminate the risk of PORC. Direct evaluation allows for a subjective diagnosis of PORC when TOF ratio=0.6 and can be assessed by an experienced clinician. Using DBS technique, the detectability of the residual neuromuscular blockade was improved, but only to TOFR=0.6.-0.7.[17] Using post tetanic stimulation, fade can be detected subjectively only when TOF ratio < 0.3 and fade after TOF stimulation can be detected when TOFR<0.4 by an experienced clinician [17, 19, 20].
Objectively (Quantitative) monitoring
Quantitative nerve stimulators can be used to objectively measure the stimulatory response and improve the reliability of TOF stimulation for assessing neuromuscular recovery. Mechanomyography is the "gold standard" among objective neuromuscular monitoring methods used in scientific research; although it is rarely used for these purposes (the device is no longer produced). It consisted of measurement of the mechanical response to a given electrical stimulus [21]. Compressomyography it is the newest method of monitoring neuromuscular transmission, and the term is not official yet (but suggested by the manufacturer). TOF-Cuff® monitor is essentially a modified cuff for Non-Invasive Blood Pressure measurement (NIBP) and neuromuscular transmission measurement. A big advantage of the device is its permanently built-in electrodes. In this method, the detected reaction is a change in cuff pressure generated by muscle contraction in response to a given stimulus [22-24]. It is possible to monitor the NM transmission in the lower limb [25]. In 1988, acceleromyography was introduced into clinical practice. Its basic principle is based on the piezoelectric effect and involves the registration of the contracting muscle acceleration after its stimulation [26]. It is a simple method to use and provides precise information about the depth of muscle relaxation. The arrival of the TOF scan monitor on the market was a great asset for anesthesiologists to work, as the device does not require calibration before the administration of non-depolarized muscle relaxants. It also measures muscle contraction in all three planes [27]. There is also an available foot and eyebrow sensor for NM transmission measurement at the tibialis posterior and facial nerves. According to Liang's 2013 study, the measurement of neuromuscular transmission using AMG is less precise than the EMG measurement, for which the TOFR measurement is often overestimated by a minimum of 0.15 [28]. In conclusion, AMG TOF>90% does not guarantee a safe extubation. An ACG TOF ratio of at least 1.00 with an additional waiting period may be necessary to exclude residual NMB [28,29]. Electromyography is another method frequently used not only in clinical trials, but also in clinical practice. This is a very simple method based on measuring the electrical response of a muscle (action potential) to a given electrical stimulus. It only requires the electrodes to be attached without fixing the accelerometer. With EMG, muscles not available for AMG, MMG or KMG, i.e. larynx and diaphragm can be monitored. EMG is more accurate than AMG [30,31]. Kinemyography, like AMG, is based on piezoelectric effect, measuring the mechanical force of muscle contraction; this method has undergone a limited number of tests and is currently rarely used in clinical practice. TOFR of 0.9 measured with KMG is comparable to a TOF ratio of 0.80 measured with EMG at the adductor pollicis muscle, but it may be as low as 0.65 or as high as 1.00. In conclusion, TOFR measured by KMG and EMG cannot be used interchangeably. Phonomyography is based on the recording of sounds emitted by contracting muscles. This is a method that allows monitoring the neuromuscular transmission in central and peripheral nerves, but is not currently applicable in clinical practice and requires further research.
Conclusion
In conclusion, neuromuscular monitoring should be applied to all patients receiving muscle relaxants because of variation in the patient's sensitivity to neuromuscular blockade. Monitoring neuromuscular function during anesthesia reduces the incidence of postoperative residual paralysis. Onset and recovery times of the vocal cord muscles and diaphragm are more resistant to the effect of nondepolarizing muscle relaxants than the adductor pollicis muscle. Depending on the degree of neuromuscular block, we have a few methods of nerve-muscle stimulation that allows observing the muscle response, but only using the TOF it is possible to assess the degree of muscle relaxation during the operation. There is no monitoring able to confirm full neuromuscular recovery, that is why when using EMG, KMG, compresomyography we need the TOF ratio >0,9 and when using AMG even 1.0 to be sure that is full neuromuscular recovery.
Conflict of Interest Statement
We declare no competing interests.
References
- Fuchs-Buder T, Meneke T, Schmartz D. Neuromuskulares Monitoring. Anaesthesist. 2001;50:129-138.
- Vimlati L, Gilsanz F, Goldik Z. Quality and safety guidelines of post anaesthesia care. Working Party on Post Anesthesia Care. Eur J Anaesthesiol. 2009;26:715-721.
- Naguib M, Brull SJ, Kopman AF, Hunter JM, Fulesdi B, Arkes HR, et al. Consensus statement on perioperative use of neuromuscular monitoring. Anesth Analg. 2018;127:71-80. [Crossref]
- Checketts MR, Alladi R, Ferguson K, Gemmell L, Handy JM, Klein AA, et al. Recommendations for standards of monitoring during anaesthesia and recovery 2015. Association of Anaesthetists of Great Britain and Ireland. Anaesthesia 2016;71:85–93.
- Nauheimer D, Geldner G. Neuromuscular monitoring: methods and machines. (AINS) Anasthesiol Intensivmed Notfallmed Schmerzther. 2008;43:374-381.
- Larsen PB, Gaetke MR, Fredensborg BB, Berg H, Engdaek J, Viby-Mogensen J. Acceleromyography of the orbicularis oculi muscle II: Comparing the orbicularis oculi and adductor pollicis muscles. Acta Anaesthesiol Scand. 2002;46:1131-1136.
- Heier T, Hetland S. A comparison of train-of-four monitoring: mechanomyography at the thumb vs acceleromyography at the big toe. Acta Anaesthesiol Scand. 1999;43:550-555.
- Kitajima T, Ishii K, Ogata H. Assessment of neuromuscular block at the thumb and great toe using accelography in infants. Anaesthesia. 1996;51:341-343.
- Saioth MJ, Fujii UY, Takahashi K, Makita K, Tanaka H, Amaha K. Recovery of post-tetanic count and train-of-four responses at the great toe and thumb. Anaesthesia.1998;53:244-248.
- Kern SE, Johnson JO, Orr JA, Westenskow DR. Clinical analysis of the flexor hallucis brevis as an alternative site for monitoring neuromuscular block from mivacurium. J Clin Anesth 1997;9:383-387.
- Merrer ML, Frasca D, Dupuis M, Debaene B, Boisson M. A comparison between the flexor hallucis brevis and adductor pollicis muscles in atracurium-induced neuromuscular blockade using acceleromyography: A prospective observational study. Eur J Anaesthesiol. 2020;37:38-43.
- Mencke T, Schmartz DT. Neuromusculares monitoring. Anaesthesist. 2013; 62:847-8461.
- Pedersen T, Viby-Mogensen J , Bang U, Olsen NV, E Jensen E, Engboek J. Does perioperative tactile evaluation of the train-of-four response influence the frequency of postoperative residual neuromuscular blockade? Anesthesiology. 1990;73:835-839.
- Cammu G, Witte JD, JanVeylder JD, Byttebier G, Vandeput D, Foubert L, et al. Postoperative residual paralysis in outpatients versus inpatients. Anesth Analg 2006;102:426-429.
- Engbaek J, Ostergaard D, Viby-Mogensen J, Skovgaard LT. Clinical recovery and train-of-four ratio measured mechanically and electromyographically following atracurium. Anaesth 1989;71:391-395.
- Capron F, Fortier LP, Racine S, Donati F. Tactile fade detection with hand or wrist stimulation using train-of-four, double-burst stimulation, 50-hertz tetanus, 100-hertz tetanus, and acceleromyography. Anesth Analg. 2006;102:15780-1584.
- Brull SJ, Silverman DG. Visual and tactile assessment of neuromuscular fade. Anesth Analg. 1993;77: 352-355.
- Trager G, Michaud G, Deschamps S, Hemmerling SM. Comparison of phonomyography, kinemyography and mechanomyography for neuromuscular monitoring. Can J Anaesth. 2006;53:130-135.
- Dullenkopf A, Horn K, Steurer M, Hess F, Welter J. Placement of TOF-Cuff® on the lower leg for neuromuscular and blood pressure monitoring during anesthetic induction for shoulder surgeries. J Anesth. 2020;34:9-85.
- Markle A, Horn K, Welter J , Dullenkopf A. An observational study comparing the performance of TOF-Cuff® with TOF-Scan monitoring during anaesthetic induction inclinical routine. Anaesthesiol Intensive Ther. 2020;52:181-186.
- Krijtenburg P, Honing G, Martini C, Olofsen E, van Elst HJ, Scheffer GJ, et al. Comparison of the TOF-Cuff® monitor with electromyography and acceleromyography during recovery from neuromuscular block. Br J Anaesth. 2019;122:22-24.
- Markle A, Graf N, Horn K, Welter JE, Dullenkopf A. Neuromuscular monitoring using TOF-Cuff® versus TOF-Scan®: an observational study under clinical anesthesia conditions. Minerva Anestesiol. 2020; 86:704-711.
- Claudius C, Viby-Mogensen J. Acceleromyography for use in scientific and clinical practice: a systematic review of the evidence. Anesthesiology. 2008;108:1117-1140.
- Chau I, Horn K, Dullenkopf A. Neuromuscular monitoring during modified rapid sequence induction: A comparison of TOF-Cuff® and TOF-Scan®. Australas Emerg Care. 2020;23:217-220.
- Liang SS, Stewart PA, Phillips S. An ipsilateral comparison of acceleromyography and electromyography during recovery from nondepolarizing neuro-muscular block under general anesthesia in humans. Anesth Analg. 2013;117:373-379.
- Capron F, Alla F, Hottier C, Meistelman C, Fuchs-Buder T. Can acceleromyography detect low levels of residual paralysis? A probability approach to detect a mechanomyographic train-of-four ratio of 0.9. Anesthesiology. 2004;100:1119-1124.
- Salminen J, van Gils M, Paloheimo M, Yli-Hankala A. Comparison of train-of-four ratios measured with Datex-Ohmeda’s M-NMT MechanoSensor and M-NMT-ElectroSensor. J Clin Monit Comput; 2016; 30;295-300.
- Jung W, Hwang M, Won YJ, Lim BG, Kong MH, Lee IOet al. Comparison of clinical validation of acceleromyography and electromyography in children who were administered rocuronium during general anesthesia: a prospective double-blinded randomized study. Korean J Anesthesiol. 2016;69:21-26.
- Stewart PA, Freelander N, Liang S, Heller G, S Phillips S. Comparison of electromyography and kinemyography during recovery from non-depolarising neuromuscular blockade. Anaesth Intensive Care. 2014;42:378-84.
- Lee W. The latest trend in neuromuscular monitoring: return of the electromyography. Anesth Pain Med. 2021;16:133-137.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.