Unilateral Closed Lip Schizencephaly with Septo‑Optic Dysplasia
- *Corresponding Author:
- Dr. Shoubhi Bhatnagar
406, Private Nimri Colony, Ashok Vihar Phase-IV, New Delhi - 110 052, India.
E-mail: shoubhi19@gmail.com
Citation: Bhatnagar S, Kuber R, Shah D, Kulkarni VM. Unilateral closed lip schizencephaly with septo-optic dysplasia. Ann Med Health Sci Res 2014;4:283-5.
Abstract
Schizencephaly is a rare malformation of the central nervous system. Both genetic and non‑genetic etiologies like prenatal infections or ischemia have been postulated. Clinical manifestations most often include varying degrees of developmental delay, motor impairment and seizures. It can be associated with septo‑optic dysplasia (SOD), optic nerve hypoplasia and absence of septum pellucidum, pachygyria, polymicrogyria, heterotopia and arachnoid cysts. We report a case of unilateral closed lip schizencephaly with SOD.
Keywords
Absence of septum pellucidum, Optic nerve hypoplasia, Schizencephaly, Septo-optic dysplasia
Introduction
Schizencephaly is a malformation of cortical development characterized by gray matter lined clefts in the cerebral cortex. It was initially described by Yakovlev and Wadsworth,[1] although descriptions of schizencephaly were first reported in the late 19th century.[2]
In a study conducted by Howe et al. (2012), 38 cases of schizencephaly were identified in 2,567,165 live births and stillbirths, giving a total prevalence of 1.48/100,000 births. It occurs more frequently in infants born to younger mothers and is often associated with septo-optic dysplasia (SOD), suggesting that the two conditions may share a common origin.[3]
The suspected cause of schizencephaly remains poorly understood. Both genetic and non-genetic etiologies such as viral infections and vascular events have been postulated. Despite the strong suspicion of a genetic etiology for schizencephaly, the majority of cases remain unexplained. Up to 70% of cases of schizencephaly are associated with absent or abnormal septum pellucidum and up to one-fourth of cases fall into the spectrum of SOD. Conversely, nearly half of the patients reported with SOD have schizencephaly.[4]
SOD, previously termed de Morsier syndrome, was first described by Reeves in 1941. It is equally prevalent in both males and females, with reported incidence of 1 in 10,000 live births.[5] The diagnosis of SOD can be made clinically when two or more features of the classical triad of (i) optic nerve hypoplasia, (ii) pituitary hormone abnormalities and (iii) midline brain defects, including agenesis of the septum pellucidum and/or corpus callosum, are present.[6] Isolated features of the triad do not fulfill the diagnostic criteria for SOD; although, debate is on-going as to whether they represent milder cases of the SOD spectrum.[7]
Case Report
Our case was a 30-year-old male patient, known case of seizure disorder since childhood. He presented to us with two episodes of generalized tonic-clonic seizures. Patient had decreased visual acuity (6/12) in both eyes since childhood.
Birth history
Our subject?s mother was 18-year-old at the time of birth and he was a full term normal delivery child with no history of birth asphyxia.
Developmental history
No history of developmental delay (Informant-Mother).
Family history
No history of any congenital anomalies in the family.
Discussion
Schizencephaly is a cerebrospinal fluid (CSF) filled cleft lined with dysmorphic gray matter extending medially from the subarachnoid space into the lateral ventricle. It results from injury involving the entire thickness of the developing hemisphere during cortical organization. The gray matter may extend into the lateral ventricle in the form of subependymal heterotopias. The cleft may be small or large, unilateral or bilateral. The anomaly may be of the open-lip or closed-lip type. Closed-lip schizencephaly is characterized by gray matter-lined lips, which are in contact with each other (type 1). Open-lip schizencephaly has separated lips and a cleft of CSF extending to the underlying ventricles (type 2).[8] Such clefts are most often found in the perisylvian region and the surrounding cortex is typically polymicrogyric.[9]
Granata et al. concluded that the severity of the motor and mental deficiencies correlated with the extent of the anatomic defect. However, the presence or severity of epilepsy did not correlate with it.[10] In 2002, Liang et al. correlated the imaging findings and clinical features of schizencephaly. They concluded that these children have variable neurological impairment and that neuroimaging is helpful in predicting developmental outcomes. A wide range of clinical phenotypes are associated with schizencephaly, depending on the size of the defect, unilateral versus bilateral defect and open-lip versus closed-lip defect.[11]
Using CT, diagnosis of Schizencephaly is often difficult particularly in case of closed lip type. However, a slight out pouching at the ependymal surface of cleft may be identified. Secondary findings such as hydrocephalus, heterotopia, polymicrogyria, subdural hygromas and arachnoid cysts may be seen. Identification of the optic nerve hypoplasia and midline brain defects including agenesis of the septum pellucidum and/or corpus callosum helps in diagnosing coexisting SOD.
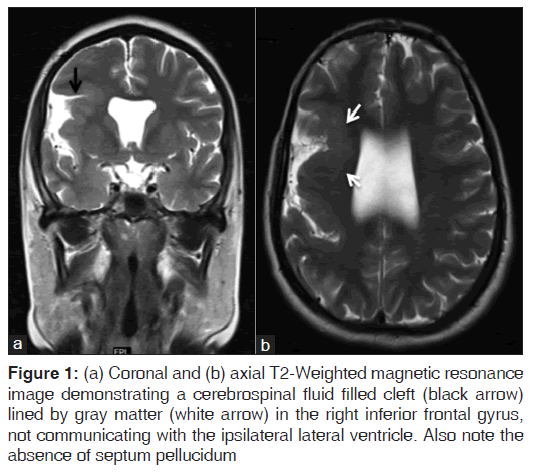
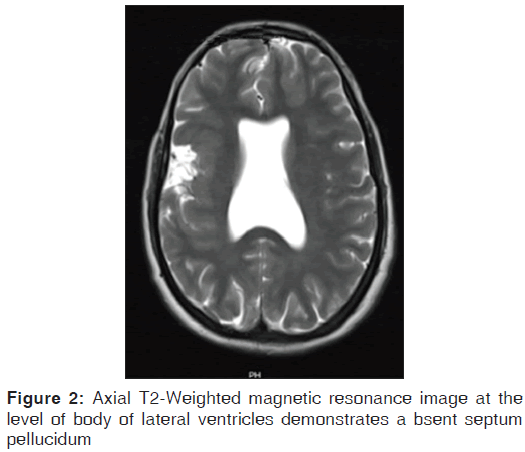
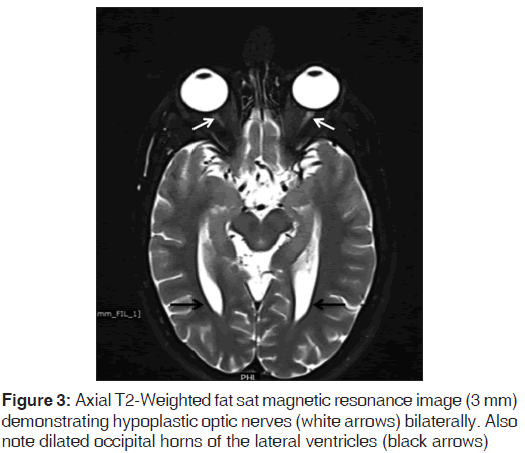
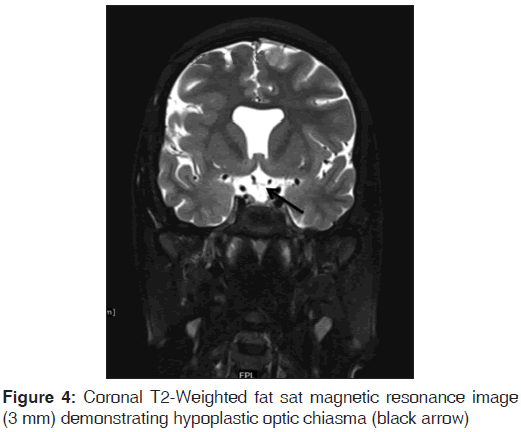
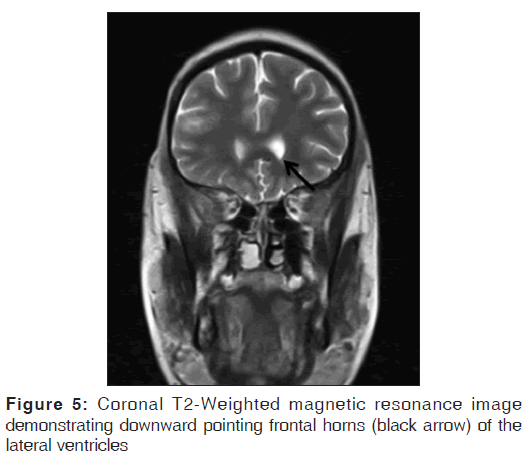
On the other hand, Magnetic resonance imaging (MRI) with its ability to differentiate gray matter and white matter depicts both the anatomy and anomaly in detail. For anatomical details, T1-Weighted images suffice. The gray matter lining the cleft, which is pathognomonic finding in Schizencephaly is easily identified using the MRI [Figure 1]. Secondary findings of gray matter heterotopias in the subcortical white matter beneath the cleft and abnormalities involving the cortex (pachygyria and polymicrogyria) can be well-appreciated. Homolateral absence of the sylvian vasculature, small medullary pyramids, low position of the fornix and thinning of the corpus callosum are findings related to absent cerebral cortex and are best demonstrated on MRI than with other studies making it the modality of choice [Figures 2-5].
Figure 1: (a) Coronal and (b) axial T2-Weighted magnetic resonance image demonstrating a cerebrospinal fluid filled cleft (black arrow) lined by gray matter (white arrow) in the right inferior frontal gyrus, not communicating with the ipsilateral lateral ventricle. Also note the absence of septum pellucidum
Differential diagnosis of schizencephaly includes subarachnoid cysts and porencephaly. Identification of the gray-matter lining of the cleft is critical in differentiating schizencephaly from porencephaly. This differentiation is important for genetic counseling since the reported incidence of brain anomalies is 5-20% in subsequent siblings of children who have anomalies of cell migration.[12]
Severe cases of schizencephaly often manifest in utero, which can be detected by prenatal ultrasound and MRI. However, many developmental and destructive lesions of the fetal brain should be considered as differential diagnosis. Prenatal detection of schizencephaly can assist in management of the pregnancy. Even if pregnancy termination is not an option, the parents will be able to prepare themselves for the inevitable clinical consequences of the defect. Given that no postnatal therapeutic options exist accurate prenatal detection becomes vital.
In case of coexistent SOD, the child may present with strabismus, nystagmus or other visual abnormalities. Clinically, the diagnosis should be suspected in newborns with hypoglycemia, jaundice, a microphallus with or without undescended testes and nystagmus with or without associated midline abnormalities such as cleft palate. In these infants, baseline endocrine tests and ophthalmology examination should be performed. Untreated hormonal abnormalities place an additional burden on a child already compromised by visual impairment and also place the patient at risk of hypoglycemia, adrenal crises and consequently death.[13]
References
- Yakovlev PI, Wadsworth RC. Schizencephalies; a study of the congenital clefts in the cerebral mantle; clefts with fused lips. J Neuropathol Exp Neurol 1946;5:116-30.
- Wilmarth WA. Presentation to the Philadelphia neurological society. J Nerv Ment Dis 1887;63 395-407.
- Howe DT, Rankin J, Draper ES. Schizencephaly prevalence,prenatal diagnosis and clues to etiology: A register-based study. Ultrasound Obstet Gynecol 2012;39:75-82.
- Barkovich AJ, Fram EK, Norman D. Septo-optic dysplasia: MR imaging. Radiology 1989;171:189-92.
- Patel L, McNally RJ, Harrison E, Lloyd IC, Clayton PE. Geographical distribution of optic nerve hypoplasia and septo-optic dysplasia in Northwest England. J Pediatr 2006;148:85-8.
- Kelberman D, Dattani MT. Genetics of septo-optic dysplasia. Pituitary 2007;10:393-407.
- Hellstr�m A, Aronsson M, Axelson C, Kyllerman M, Kopp S, Steffenburg S, et al. Children with septo-optic dysplasia-How to improve and sharpen the diagnosis. Horm Res 2000;53 Suppl 1:19-25.
- Oh KY, Kennedy AM, Frias AE Jr, Byrne JL. Fetal schizencephaly: Pre- and postnatal imaging with a review of the clinical manifestations. Radiographics 2005;25:647-57.
- Barkovich AJ, Norman D. MR imaging of schizencephaly. AJR Am J Roentgenol 1988;150:1391-6.
- Granata T, Farina L, Faiella A, Cardini R, D'Incerti L, Boncinelli E, et al. Familial schizencephaly associated with EMX2 mutation. Neurology 1997;48:1403-6.
- Liang JS, Lee WT, Peng SS, Yu TW, Shen YZ. Schizencephaly: Correlation between clinical and neuroimaging features. Acta Paediatr Taiwan 2002;43:208-13.
- Dobyns WB, Stratton RF, Greenberg F. Syndromes with lissencephaly. I: Miller-Dieker and Norman-Roberts syndromes and isolated lissencephaly. Am J Med Genet 1984;18:509-26.
- Webb EA, Dattani MT. Septo-optic dysplasia. Eur J Hum Genet 2010;18:393-7.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.