Vascularized Fibula Flaps for Mandibular Reconstruction: An Institutional Audit
Citation: Okoturo E, Osasuyi A, Opaleye O, Wemambu J, Alonge M, Somoye O, et al. Vascularised Fibula Flaps for Mandibular Reconstruction: An Institutional Audit. Ann Med Health Sci Res. 2017; 7:46-51.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Background: Mandibular defects, when left untreated results in mandibular deviation, malocclusion, patient restriction to soft diet and cosmetic anomaly. For decades, osseous vascularised flaps have been used for reconstruction of the mandible with the vascularised fibula flap (VFF) remaining the commonly used osseous free flap, reasons ranging from its adequate bone and pedicle length to its receptive dental implant placement quality. This report considers a modest use of the VFF for mandibular reconstruction following ablative tumour surgery in a limited resource environment. Subjects and Method: This study data represents a cohort of subjects from the maxillofacial, head and neck surgical oncology division of the study institution that underwent mandibular reconstruction with VFF. Data collated comprised; demographic, histopathology, types of mandibulectomy, defect classification, types of vascularised fibula flap reconstruction, bone and pedicle length with or without skin paddle dimension, tourniquet and ischaemic time, overall surgery time, flap outcome, clinical outcome and complications following reconstruction. Result: A total of 27 patients had consecutive mandibular resection over the study period. Of these, 8 patients had VFF reconstruction done. Their age range was between 24-62 years with an average age of 39.4 ± 12.87 years. The predominant histopathological diagnoses were solid/multicystic ameloblastoma with 6 (75%) cases. The predominant defect was LC (Jewer’s classification) related with 5 (62.5%) cases. The average donor bone length was 10.52 ± 3.43 cm. The average pedicle length was 7.55 ± 1.33 cm. A total of 4 (50%) flaps were lost while 4 (50%) flaps were viable. Of the 4 reconstructions with viable flaps, 2 (50%) had good clinicoaesthetic outcomes while the other 2 (50%) had acceptable outcomes. Conclusion: This small sampled study tended to suggest a steeper learning curve with a high failure rate and moderate clinical outcome. Emphasis for start-ups in these environments should be towards building a team across surgical training and nursing care.
Keywords
Vascularised Fibula Flap, Mandible, Reconstruction
Introduction
Mandibular defects, when left untreated result in mandibular deviation on mouth opening, malocclusion with patient restriction to soft diet and cosmetic anomaly [1].
Over the last two-three decades, mandibular reconstruction using reconstruction plates with (or without) non-vascularised bone graft has overtly been replaced with composite or osseous vascularised flap surgery [2-4].
For decades, osseous vascularised flaps has been used for mandibular reconstruction with the vascularised fibula flap (VFF) remaining the commonly used osseous free flap. Reasons for this resonate with its adequate bone and pedicle length to its receptive dental implant placement quality [4-7].
Taylor et al. and Gilbert in the 1970s first described the harvesting technique of the VFF. However, not after Hidalgo’s publication in 1989 did it have a widespread use for mandibular reconstruction [2,8,9]. VFF is based on the peroneal artery and its venae commitantes and can be harvested either as a vascularised osseous or osseo-cutaneous flap with a sensate variant [10,11]. Based on the length of bone that can be harvested, the VFF remains the only flap suitable for reconstruction of total or subtotal mandibular defects [10].
Reconstruction of mandibular defect at our center comprises the use of reconstruction plate with or without non-vascularised iliac crest graft and VFF.
This report considers a modest use of the VFF for mandibular reconstruction following ablative tumour surgery in a limited resource environment.
Patients and Methods
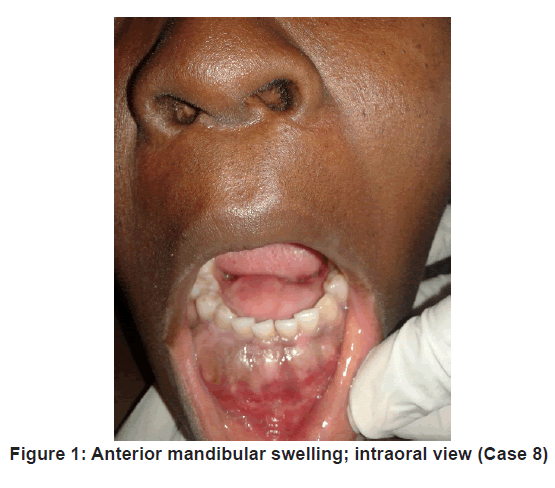
This study data represents a cohort from the maxillofacial, head and neck surgical oncology division of the study institution that underwent mandibular reconstruction with VFF between the years 2013 – 2016, an example shown in Figure 1.
Data collated included; demographic data, histopathology, types of mandibulectomy, defect classification, types of vascularised fibula flap reconstruction, bone and pedicle length with or without skin paddle dimension, tourniquet and ischaemic time, overall surgery time, flap outcome, clinical outcome and complications following reconstruction.
The head and neck surgical oncology unit was divided into two teams where possible, which performed simultaneous mandibular resection and VFF harvesting with vascular anastomoses. The indications for VFF reconstruction were inability to bridge hard tissue defect and soft tissue coverage with the conventional technique (iliac crest bone graft) and also having a relative comparative advantage over conventional technique.
Anaesthetist assessed patient’s fitness for surgery and preoperative doppler ultrasonography of the less dominant leg was performed to assess peroneal circulation.
Mandibular resections were classed as segmental mandibulectomy (with or without condylectomy), hemimandibulectomy and subtotal mandibulectomy.
Defect classifications were classed according to Jewer et al. or HCL classification [11]. It was defined as follows; bone defect involving the entire central segment including both canines was designated (C). The lateral segment excluding the condyle was designated (L) while the lateral segment inclusive of the condyle was designated (H) or hemi-mandibulectomy where appropriate. With these, three single and five combined designates were therefore possible: C, L, H, LC, HC, LCL, HCH and HH [11]. The classification was further modified to incorporate soft tissue defect with “t” designating tongue defect, “m” designating mucosa defect and “s” designating skin defect [12].
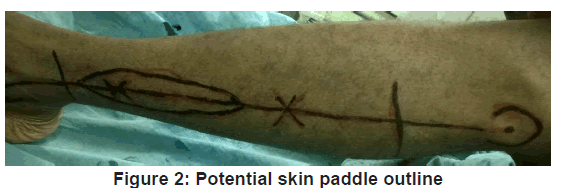
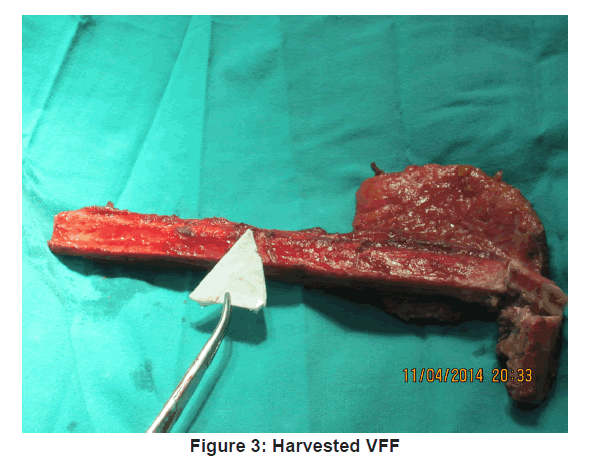
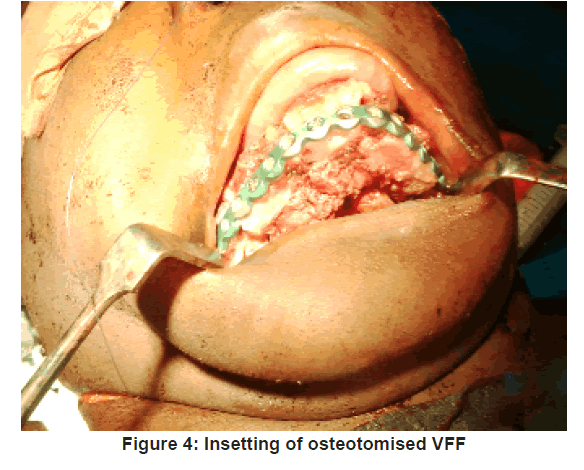
The VFF skin markings were made. A hand held ultrasonic doppler (Datascope IABP 8MHz) was used to identify at least one perforator along the axis of the potential skin paddle. The skin paddle outline was then traced around the perforator as shown in Figure 2 and its predetermined dimension outlined. Fasciocutaneous fibula flap harvesting was done using the standard lateral approach as described by Gilbert [9] under tourniquet control of 300 mmHg. The harvested fibula was osteotomised as appropriate and insetted to produce appropriate anatomical contour for the mandibular defect. The harvested fibula with its osteotomised segments was fixed together and to the mandibular segment, using 3.0 mm locking (KLS Gebrueder martin) recon plate [Figure 3 and 4].
The peroneal artery and its dominant venae commitantae were anastomosed end to end, to the facial artery and vein using 9.0 mm Ethicon nylon sutures (Ethicon USA) at X 4 magnification using an operating microscope (Zeiss OPMI Vario / S88). Donor site was grafted with a split thickness skin graft.
Intraoperatively, patient underwent standard physiological monitoring of oxygenation; end tidal CO2, circulation, core temperature and urine output.
The study institution’s head and neck free flap monitoring protocol was instituted with osseocutaneous flap vitality assessed based on skin paddle colour, texture, and capillary refill (>3 seconds). Hand held ultrasonic doppler was used to monitor arterial flow for osseous flaps. Patients were nursed in ICU for the first 3 days and then high dependence ward admission for additional 6-9 days before ward discharge.
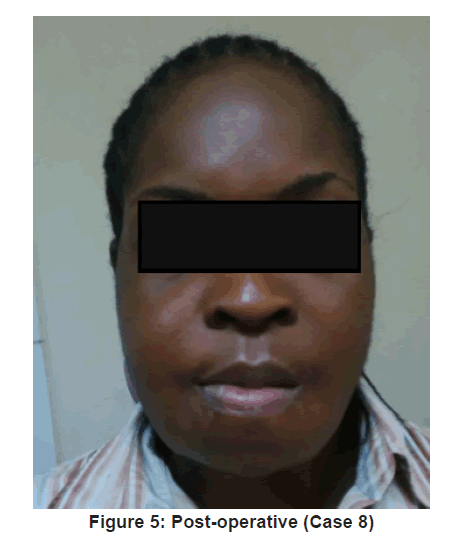
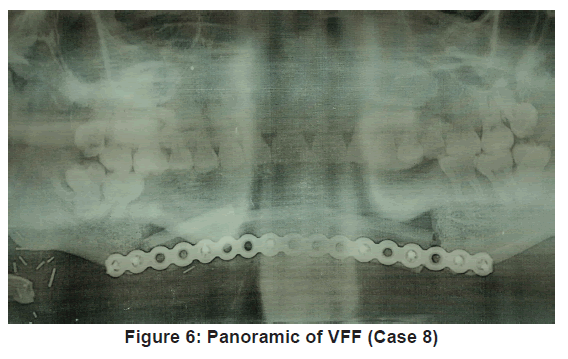
Flap outcome was evaluated periodically over 12 months, post reconstruction [Figure 5 and 6]. Vascularised fibula flap was adjudged successful when a postoperative panoramic image showed complete consolidation of the bone graft [13]. Clinical outcome was evaluated based on adequate mouth opening and swallowing. It was adjudged “excellent” or “good” when no alteration in mouth opening or limitation during swallowing occurred. It was considered “acceptable” when mouth alteration and swallowing limitations were mild to moderate. It was “poor” when mouth alteration and swallowing limitations were significant. Aesthetic outcome were classed “good” when surgeon and patient did not find any alteration to site of reconstruction, “acceptable” when alterations were mild to moderate and “poor” when alterations were significant [14].
Postoperative complications, adjudged as flap and donor site related, were reviewed and analysed. The flap related complications were classed as total or partial flap loss (TFL or PFL), flap skin dehiscence (FSD) and flap skin partial necrosis (FSPN). Total flap loss was defined as complete necrosis of flap tissue while partial flap loss was defined as complete necrosis of skin paddle. FSD and FSPN are self-explanatory. The donor site related complication comprised; donor site infection (DSI) and donor site wound dehiscence (DSWD). Flap viability and complications of patients who expired at less than 12 months post reconstruction, were included in the analysis.
Data were analysed using IBM SPSS (statistical package for social science) for Mac OS version 21.0. Percentages, mean, standard deviation and predictor variables analyses (multivariate analysis) for age, gender, histopathologic diagnosis, type of mandibular resection, defect classification, skin paddle dimension, tourniquet and ischaemic time, overall surgery time, flap outcome (clinical and aesthetic) and postoperative complications were analysed. A test of significance was set at; p value < 0.05. Ethical approval was obtained for this retrospective study according to the rules governing the institutional review board. All participants signed an informed consent agreement for blinded photographs.
Results
Demographic data
A total of 27 patients had consecutive mandibular resection over the study period. All cases had mandibular defects that met the study inclusion criteria. Of these, 8 patients had VFF reconstruction done. The other 19 cases had recon plates placed. Of these 19 cases, 4 cases had recon plates to facilitate secondary VFF reconstruction, 13 cases could not have VFF done due to financial constraints while 2 others opted for plate only reconstruction due to anxiety related reasons. Of the 8 cases that had VFF, 3 were females and 5 were males. Their age range was between 24-62 years with an average age of 39.4 ± 12.87 years.
Histopathology, defect and tissue dimension data
The predominant diagnoses were solid/multicystic ameloblastoma with 6 (75%) cases and ossifying fibroma, being the only other diagnoses. The predominant defect was LC related with 5 (62.5%) cases; of which LCL was 3 (37.5%) cases. 4 (50%) cases had osteocutaneous flap reconstruction. The donor bone length range was between 6.8-18.2 cm with an average donor bone length of 10.52 ± 3.43 cm. The pedicle length range was between 4.8–9.4 cm with an average pedicle length of 7.55 ± 1.33 cm. Case No. 2 required pedicle lengthening, end-to-end vascular anastomoses (using external jugular vein). On number of osteotomies performed, 4 cases (50%) had 2 osteotomies with 3 fragments while 2 cases (25%) had 1 osteotomy with 2 segments. 1 (12.5%) case each, had 0 osteotomy and 3 osteotomies with 1 and 4 segments respectively.
Surgery times data
The TT ranged between 150 - 205 minutes with an average TT of 171.38 ± 19.95 minutes. The IT ranged between 151-310 minutes with an average IT of 220.13 ± 56.90 minutes. The TST ranged between 14.00hrs – 19.50 hrs with an average TST of 16.00hrs ± 1.94hrs.
Complications and outcomes data
A total of 4 (50%) flaps were lost while 4 (50%) flaps were viable. Of the 4 VFF that failed, 3 failures were adduced to arterial thrombosis based on capillary refill test becoming negative for 2 VFF and Doppler monitoring becoming negative for an additional osseous VFF. While one failure was adduced to venous thrombosis as capillary refill test was initially rapid (< 3 secs) before eventual becoming negative. Of the 4 reconstructions with viable flaps, 2 (50%) had good clinicoaesthetic outcomes while the other 2 (50%) had acceptable outcomes. In the immediate postoperative period, 2 patients died from medical related causes. All other data including postoperative complications are as detailed in Tables 1 and 2.
| No. | Gender | Age | Diagnosis | Defect* Classification | Flap Composition | Flap Outcome | Clinico-aesthetic Outcome |
|---|---|---|---|---|---|---|---|
| 1. | M | 53 | A | L | Osteocutaneous | Failed | - |
| 2. | F | 34 | RA | LCH | Osseous | Viable | Acceptable |
| 3. | M | 62 | A | H | Osteocutaneous | Failed | - |
| 4. | M | 24 | A | HC | Osseous | Viable | Good |
| 5. | M | 29 | A | LCL | Osteocutaneous | Failed | - |
| 6. | M | 45 | RA | LCL | Osseous | Failed | - |
| 7. | F | 34 | OF | LC | Osseous | Viable | Acceptable |
| 8. | F | 34 | OF | LCL | Osteocutaneous | Viable | Good |
*= Jewer et al Classification15, A=Ameloblastoma, RA=Recurrent Ameloblastoma, OF=Ossifying Fibroma.
Table 1. Clinico-demographic data
| No. | Fibula Length (cm) | Size of Skin Paddle (cm) | Pedicle length (cm) | TT (hrs) | TT (hrs) | TST (hrs) | No of Osteotomies | Complications |
|---|---|---|---|---|---|---|---|---|
| 1. | 6.8 | 2×2 (flap monitoring) | 9.4 | 2.51 | 1.3 | 15.20 | 0 | TFL |
| 2. | 18.2 | - | 4.8 | 2.35 | 5.0 | 19.50 | 3 | DSI |
| 3. | 8.8 | 5×3 | 8.2 | 2.30 | 3.0 | 15.30 | 1 | TFL* |
| 4. | 9.5 | - | 7.4 | 3.25 | 5.0 | 18.30 | 2 | DSWD* |
| 5. | 11.0 | 6×4 | 8.4 | 3.05 | 3.4 | 16.10 | 2 | TFL |
| 6. | 11.2 | - | 7.2 | 3.10 | 3.3 | 14.20 | 2 | DSI |
| 7. | 8.2 | - | 7.2 | 2.40 | 3.0 | 14.00 | 1 | DSWD |
| 8. | 10.2 | 6×4 | 7.8 | 2.35 | 3.3 | 15.40 | 2 | FSD |
* = Death, TFL=total flap loss, PFL=Partial flap loss, FSD= flap skin dehiscence, FSPN=flap skin partial necrosis, DSI= Donor site infection, DSWD= Donor site wound dehiscence.
Table 2. Relevant clinical data and complications
Predictors of complications and flap outcomes
Variables with impact on complications and flap outcomes were analysed using univariate analysis. Age was the only variable with statistical significant impact on flap outcome. The remaining analyses are as shown in Table 3.
| Variable | P-Value |
|---|---|
| Age | < 0.001* |
| < 50 | |
| > 50 | |
| Gender | 0.46 |
| Female | |
| Male | |
| Defect | 0.76 |
| <LC | |
| >LC | |
| Flap type | 0.66 |
| OF | |
| OCF | |
| TST | 1.00 |
| < 13hrs | |
| > 13 hrs | |
| Osteotomies | 1.00 |
| < 2 | |
| > 2 |
* Statistically significant
OF= Osseous flap, OCF = Osteocutaneous flap
Table 3. Predictors of flap outcomes
Discussion
The vascularised fibula flap is reported to provide the best option for mandibular reconstruction by availing good length of bone of about 25 cm with ability for multiple osteotomies at little blood supply compromise 15. Based on the peroneal artery and its venae commitantes, it can provide a pedicle length of about 15 cm with good vessel calibre of between 2-3 mm in diameter [3]. The potential advantages of VFF comprise its bone strength with high-pressure resistance, high content of cortical bone suitable for dental implant placement and multiple osteotomisation to allow for mandibular adaptation and contouring [14-16].
Of the 27 patients that had mandibular resections requiring reconstructions, 8 (29.6%) were able to have VFF reconstruction. This low number was mostly due to financial constraints and partly to apprehension on the part of 2 patients. The average age of 39 years in this study was less than that of other reports. This was due to the absence of malignancy in these studies, which affects older patients [14,17].
In the present sample, the predominant mandibular defect characterisations were 5 (62.5%) bi-mandibular defects characterised as 3 LCL and 1 each of LC and HC, with the remaining being ipsilateral defects. This was in consonance with reports in the literature, which adjudged LCL and bi-mandibular defects as the commonest defects 17,18. The reason adduced was the causal relationship between bi-mandibular defects and ablative tumour surgery, an occurrence also exhibited in this study. On the converse, lateral defects are purportedly caused by non-tumour/oncology related incidents [17]. In continuum, reports from our earlier study (probably the only data from region of study institution), which audited 34 mandibular defects, reported class HCL (a larger bi-mandibula defect) as the predominant defect. While we shared the reported reason earlier adduced for causation of bi-mandibular defect, the peculiar late case presentation, synonymous with our environment, accounted for our larger bi-mandibular defect in HCL [18].
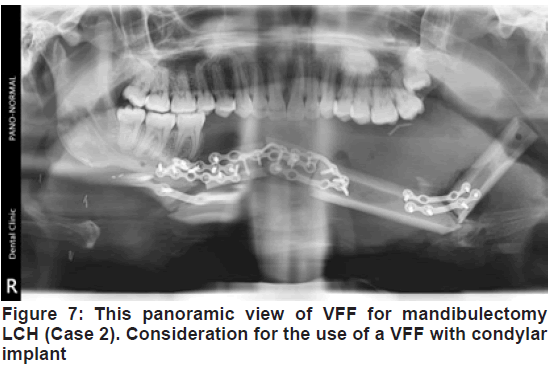
After harvesting and osteotomization, the average transferred fibula and pedicle length of the present sample were 10.5 cm and 7.5 cm respectively. This fell within the range of other reports [11,14,17]. In addition, the predominant bi-mandibular defect and its 3-dimensional complexity were brought to bear as most of the cases had 2 osteotomies with 3 segments. This was similar to other studies that reported an incidence of 2 osteotomies [17]. Of particular mention was the reconstruction of the LCH defect of case 2 [Figure 7]. While the clinical outcome was adjudged acceptable, radiologically it appeared beyond the indication for VFF alone. A combined VFF with condylar implant may have been appropriate.
All 8 (100%) cases had postoperative complications comprising 4 (50%) cases with total flap loss and 2 (25%) cases with donor site infection and wound dehiscence respectively. These postoperative complications were far higher than in other reports, an occurrence adduced to the small number of cases, and being the first set of cases, which captured the learning curve period [17].
VFF is usually harvested with an accompanying fasciocutaneous component or a skin paddle, which is vascularised in 90-95% of the time, by septocutaneous perforators of the peroneal vessels [15]. While the reliability of its skin paddle has been questioned by earlier researchers, later reports have detailed its reliability to be more than 90% [14,17,20]. However, our study was unable to weigh in on this discus, as 3 out of 4 of the osteocutaneous flaps failed. Reason for this adduced more to the difficult learning curve than the variability of cutaneous perforators.
With respect to pre-surgical studies, while the consensus on peroneal assessment is magnetic resonance angiogram, the cost of this makes it difficult to achieve in less opportune environment like the study environment. Thus, an alternative in ultrasonic doppler was used. Its use is supported in the literature and was employed to success in this study [14,19,21].
In terms of predictors of complications and outcomes, age of >50 years, was the only variables that reported any statistical significance (p<0.0001). While longer operation times have been associated with the development of postoperative complications, researchers are yet to attain a consensus on increasing age and development of postoperative complications [22,23].
Conclusion
VFF is an ideal donor for mandibular reconstruction because of its adequate bone length and distant site that allows for a twoteam approach. Its skin paddle can be used to repair intraoral lining.
This small sampled study, tend to suggests a steeper learning curve with high failure rate and moderate clinical outcome. Emphasis for start-ups in these environments should be towards building a team across surgical training and nursing care.
REFERENCES
- Schliephake H, Neukam FW, Schmelzeisen R. Long-term quality of life after ablative intraoral tumor surgery. J Craniomaxillofac Surg. 1995;23:243
- Hidalgo DA. Fibula free flap. A new method of mandible reconstruction. Plast Reconstr Surg. 1989; 84:71.
- Hidalgo DA, Rekow A. A review of 60 consecutive fibula free flap mandible reconstructions. Plast Reconstr Surg. 1995; 96:585–602.
- Vayyada H, Mola F, Menderes A. Surgical management of ameloblastoma in the mandible: Segmental mandibulectomy and immediate reconstruction with free fibula or deep circumflex iliac artery flap. J Oral Maxillofac Surg. 2006; 64:1532.
- Nkenke E, Vairaktaris E, Stelzle F. Osteocutaneous free flap including medial and lateral scapular crests: Technical aspects, viability, and donor site morbidity. J Reconstr Microsurg. 2009; 25:545.
- Eckardt A, Swennen G, Barth EL. Long-term results after mandibular continuity resection in infancy: The role of autogenous rib grafts for mandibular restoration. J Craniofac Surg. 2006;17:255.
- Ferrari S, Copelli C, Bianchi B, Ferri A, Poli T. Rehabilitation with endosseous implants in fibula free-flap mandibular reconstruction: A case series of up to 10 years. Journal of Cranio-Maxillo-Facial Surgery. 2013; 41:172-178.
- Taylor GI, Miller DH, Ham FJ. The free vascularised bone graft: A clinical extension of microvascular techniques. Plast Reconstr Surg. 1975; 55:533.
- Gilbert A. Vascularised transfer of the fibular shaft. Int J Microsurg. 1979; 1:100
- Urken ML, Cheney ML, Sullivan MJ, Biller HF. Atlas for regional and free flap for head and neck reconstruction. 1st Ed, Raven press, New York. 1995; 292.
- Jewer DD, Boyd JB, Manktelow RT, Zuker RM, Rosen IB. Orofacial and mandibular reconstruction with the iliac crest free flap: a review of 60 cases and a new method of classification. Plast Reconstr Surg. 1989; 84:391-403.
- Hidalgo DA, Pusic AL. Free-flap mandibular reconstruction: A 10-year follow-up study. Plast Reconstr Surg. 2002; 110:438–451.
- Van Gemert JT, Van Es RJ, Rosenberg AJ, Van der Bilt A, Koole R, Van Cann EM. Free Vascularized Flaps for Reconstruction of the Mandible: Complications, Success, and Dental Rehabilitation. J Oral Maxillofac Surg. 2012; 70:1692-1698.
- González-García R, Naval-Gías L, Rodríguez-Campo FJ, Muñoz-Guerra MF, Sastre-Pérez. Vascularized free fibular flap for the reconstruction of mandibular defects: Clinical experience in 42 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008; 106:191-202.
- Wei FC, Seah CS, Tsai YC, Liu SJ, Tsai MS. Fibula osteoseptocutaneous flap for reconstruction of composite mandibular defects. Plast Reconstr Surg. 1994; 93:294–306.
- Gbara A, Darwich K, Li L, Schmelzle R, Blake F. Long-term results of jaw reconstruction with microsurgical fibula grafts and dental implants. J Oral Maxillofac Surg. 2007; 65:1005.
- López-Arcas JM, Arias J, Del Castillo JL, Burgueño M, Navarro I, Morán MJ, et al. The Fibula Osteomyocutaneous Flap for Mandible Reconstruction: A 15-Year Experience. J Oral Maxillofac Surg. 2010; 68:2377-2384.
- Okoturo E, Osasuyi A. Maxillo-mandibula defect reconstruction. An institution’s experience in a limited opportunity environment. Nig J Clin Med. 2015; 7: 24-34.
- Young DM, Trabulsy PP, Anthony JP. The need for preoperative angiography in fibula free flaps. J Reconstr Microsurg. 1994; 10:283-287.
- Wong CH, Tan BK, Wei FC, Song C. Use of the soleus musculocutaneous perforator for skin paddle salvage of the fibula osteoseptocutaneous flap: anatomical study and clinical confirmation. Plast Reconstr Surg. 2007; 120:1576–1584.
- Futran ND, Stack BC, Zaccardi MJ. Preoperative color flow Doppler imaging for fibula free tissue transfers. Ann Vasc Surg. 1998; 12:445-450.
- Grammatica A, Piazza C, Parderno A, Taglietti V, Maregoni A, Nicolai P. Free Flaps in Head and Neck Reconstruction after Oncologic Surgery: Expected Outcomes in the Elderly. Otolaryngol Head Neck Surg. 2015; 152: 796-802.
- Ozkan O, Ozgentas HE, Islamoglu K, Boztug N, Bigat Z, Dikici MB. Experiences with microsurgical tissue transfers in elderly patients. Microsurgery 2005; 25:390–395.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.