Verification of Entrance Dose Measurements with Thermoluminescent Dosimeters in Conventional Radiotherapy Procedures Delivered with Co‑60 Teletherapy Machine
- *Corresponding Author:
- Mr. Evwierhurhoma OB
Department of Radiotherapy, Eko Hospitals, Ekocorp Plc, Ikeja, Lagos, Nigeria.
E-mail: benomus@yahoo.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
Background: The use of in vivo dosimetry with thermolumiscent dosimeters (TLDs) as a veritable means of quality control in conventional radiotherapy procedures was determined in this work. Aim: The objective of this study was to determine the role of in vivo dosimetry with thermoluminescent dosimeters (TLDs) as part of quality control and audit in conventional radiotherapy procedures delivered with Co‑60 teletherapy machine. Subjects and Methods: Fifty‑seven patients with cancers of the breast, pelvis, head and neck were admitted for this study. TLD system at the Radiation Monitoring and Protection Centre, Lagos State University, Ojo, Lagos‑Nigeria was used for the in vivo entrance dose readings. All patients were treated with Co‑60 (T780c) teletherapy machine at 80 cm source to surface distance located at Eko Hospitals, Lagos. Two TLDs were placed on the patient surface within 1 cm from the center of the field of treatment. Build‑up material made of paraffin wax with a density of 0.939 g/cm3 and a thickness 0.5 cm was placed on top of the TLDs. A RADOS RE 200 TLD reader was used to read out the TLDs over 12 s and at a temperature of 300°C. Results: The results showed that there was no significant difference between the expected dose and measured dose of breast (P = 0.11), H and N (P = 0.52), and pelvis (P = 0.31) patients. Furthermore, percentage difference between expected dose and measured dose of the three treatment sites were not significantly different (P = 0.11). More so, 88.9% (16/18) treated breast, 91.3% (21/23) pelvis, and 86.7% (13/15) H and N patients had percentage deviation difference less than 5%. In general, 89.3% (50/56) patients admitted for this study had their percentage deviation difference below 5% recommended standard limit. Conclusion: The values obtained establish that there are no major differences from similar studies reported in literature. This study was also part of quality control and audit of the radiotherapy procedures in the center as expected by national and international regulatory bodies.
Keywords
Co-60 machine in vivo dosimetry, Conventional radiotherapy, Entrance dose, Thermoluminescent dosimeters
Introduction
Radiotherapy is a multidisciplinary specialty using complex equipment and procedures for assessment, planning, and delivery of the treatment. International Commission on Radiation Units and Measurements (ICRU) [1] recommends target dose uniformity within ± 5% of the dose delivered to a well-defined prescription point within the target. Modern photon beam radiotherapy is done with a variety of beam energies and field sizes under one of two set-up conventions: A constant source to surface distance (SSD) for all beams or an isocentric set-up with a constant source to axis distance. It is universally recognized that quality assurance (QA) is vital to overall radiotherapy process to ensure the achievement of safe and effective treatment.[1-3] The goal of a radiotherapy procedure is to deliver maximum dose to eradicate a tumor while at the same time minimizing the radiation exposure to healthy tissues.
In vivo dosimetry is the procedure adopted to monitor the radiation dose delivered to a patient during radiation therapy.[2,4] It allows comparison of prescribed and delivered doses and thus provides a level of radiotherapy QA that supplements portal films and computational double checks. The ultimate check of the actual dose delivered to a patient in radiotherapy can only be achieved using in vivo dosimetry.[5,6] In vivo dosimetry can be done by putting dosimeters on the patient’s skin or in natural cavities.[4] It is usually performed to detect errors in individual patient’s and core procedures to evaluate the quality of specific treatment techniques or to evaluate the dose in situations in which the dose calculation is inaccurate or not possible.[2,4,7]
In vivo dosimetry can be divided into three classes: Entrance dose measurements, exit dose measurements, and intracavitary dose measurements.[4,8,9] Entrance dose measurements serve to check the output and performance of the treatment apparatus as well as the accuracy of patient set-up. Exit dose measurements serve, in addition, to check the dose calculation algorithm and to determine the influence of shape, size, and density variations of the body of the patient on the dose calculation procedure; a variety of detectors, including thermoluminescent dosimeters (TLD), silicon diodes, and new detectors such as metal oxide silicon field-effect transistors are currently available for in vivo dosimetry.[2,10-15] The choice between these techniques may depend on many factors such as availability, intrinsic characteristics of the detector type, measurement type, training of personnel, financial considerations, and, of course, personal preference.[4,12-14]
This work reports a study to test the applicability of a TLD system for performing in vivo entrance dose measurements in cobalt teletherapy machine.The use of TLD for in vivo dosimetry had been reported by many authors as an effective means of entrance dose verification in radiotherapy.[16-19]
In vivo dosimetry was carried out on various cancer patients at the radiotherapy department of Eko Hospitals, Lagos-Nigeria.
The aim of this study is to verify whether a correct dose is actually being delivered to the tumor and also detect errors in individual treatment sessions that may arise in equipment malfunctioning and human mistakes.
Subjects and Methods
Fifty-six patients with different types of cancer diseases were randomly selected and admitted for this study after obtaining due clearance from the ethical committee of the hospital. Admission of patients for this study was based on the cancer distributions reported for treatment in the center and each patient consent was sought before measurements were taken. The most common cancer types featuring in the center are: Breast, pelvis (prostate and cervical), and head and neck (H and N). The TLD chips were divided into three groups: Breast (18), pelvis (23), and H and N (15). The TLD system available at the Radiation Monitoring and Protection Centre of the Lagos State University, Ojo, Lagos was used for the study. The choice of LiF: MgTi is based on its special characteristics which include: Energy independence, directional independence, small size, tissue equivalence, linearity within the energy range of interest, reusability, and availability.
All patients were treated using gamma 60Co (T780c) radiation at SSD 80 cm located at Eko Hospitals, Ikeja, Lagos. Two TLDs were placed on the patient surface within 1 cm from the center of the field of treatment. Build-up material made of paraffin wax with a density of 0.939 g/cm3 and a thickness 0.5 cm was placed on top of the TLDs. An RADOS RE 200 TLD reader was used to read out the TLDs over 12 s and at a temperature of 300°C within the fields of treatment. The doses were averaged, and the mean dose of all patients measured. SSD and build-up correction factors were determined. The measured doses are the product of results of readings, calibration, and correction factors. Percentage differences between the measured and expected doses were calculated.
Thermoluminescent readouts were carried out using an RADOS RE 2000 TLD reader with a linear heating rate of 8 C/s. A nitrogen gas generator supplied heat to the TLD reader. Readouts were taken within 12 s and at a temperature of 300. An oven and a furnace were used for annealing procedures of the LiF: MgTi. The annealing procedure used consists of two subsequent annealing: 1 h at 400°C and 2 h at 100°C
Statistical analysis
The expected doses, measured doses, dose deviations, and percentage mean deviations were recorded as means (standard deviation [SD]). Statistical analyses for all the data were performed using Statistical Package for Social Sciences (SPSS) version 20 (Armonk, NY, USA). A one-way analysis of variance (ANOVA) was used to study the relationships that exist among the parameters. Tukey’s post-hoc multiple comparison method was further used to test the statistically significant relationship among the groups. P <0.05 was considered statistically significant.
Results
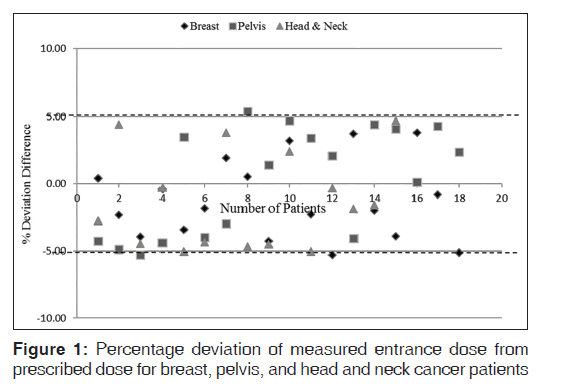
Table 1 presents mean (SD) of expected doses, measured dose, dose deviation, and percentage deviation differences of a group of patients treated for breast, pelvis, and H and N cancer. The result values are presented in mean (SD). There was no significant difference in percentage deviation of measured dose from prescribed dose for breast and pelvis patients (P = 1.00), breast and H and N patients (P = 0.17), and pelvis and H and N patients (P = 0.18). Also no significant difference between percentage deviations of measured and prescribed doses of breast, pelvis, and H and N patients (P = 0.11). Figure 1 represents the relationship between percentage deviation differences of measured entrance doses from prescribed doses for breast, pelvis, and H and N patients. The dotted lines on the figure indicate the ±5% recommended limit.[1,4]
| Treatment site | n | |||||
|---|---|---|---|---|---|---|
 (s) (Gy) (s) (Gy) |
 (s) (Gy) (s) (Gy) |
 (s) (Gy) (s) (Gy) |
 (s) (s) |
 <5% <5% |
||
| Breast | 18 | 1.25 (0) | 1.27 (0.04) | 0.02 (0.03) | 1.22 (2.97) | 88.9 |
| Pelvis | 23 | 1.25 (0.40) | 1.26 (0.38) | 0.01 (0.05) | 1.30 (3.70) | 91.3 |
| H and N | 15 | 1.12 (0.33) | 1.11 (0.32) | 0.01 (0.05) | 0.83 (3.94) | 86.7 |
| Total | 56 | 1.20 (0.30) | 1.20 (0.29) | 0.00 (0.25) | 0.40 (3.67) | 89.3 |
n: Number of treatment site,  : Expected dose,
: Expected dose,  : Mean of measured dose,
: Mean of measured dose,  : Mean dose difference,
: Mean dose difference,  : Percentage mean deviation,
: Percentage mean deviation,  : Percentage of measurements
within the ±5% tolerance level and s is the SD. SD: Standard deviation, H and N: Head and neck
: Percentage of measurements
within the ±5% tolerance level and s is the SD. SD: Standard deviation, H and N: Head and neck
Table 1: Deviation of expected dose from measured dose of breast, pelvis, and H and N patients
Discussion
This study was designed to investigate the percentage difference between expected dose and measured dose of different patients scheduled for radiotherapy in the center. Table 1 presents the average expected dose, measured dose, dose deviation, percentage deviation differences between the expected and measured doses of breast, pelvis, and H and N, respectively. A positive value indicates that the measured value was greater than the expected dose; while a negative value indicates that the measured dose is less than the expected dose. From the results, 88.9% (16/18) patients treated for breast were below ± 5% recommended dose limits which is in good agreement with Fiorino et al.,[20] Cozzi and Fogliata-Cozzi,[21] and Aweda et al.[22] Patients with pelvis had 91.3% (21/23) below recommended dose tolerance limit which is also in good agreement with Ferguson et al.[23] and Meijer et al.[24] values. Also, 86.7% (13/15) of the patients recruited for the H and N treatments had their obtained values below recommended dose limit, which is in agreement with Fiorino et al[20] and others.[22,24] In general, 89.3% (50/56) of the patients admitted for this study were below 5% recommended dose limit. ANOVA was used to test the relationship between the results of the treated sites; there was no significant difference in percentage deviation of measured dose from prescribed dose of breast and pelvis patients (P = 1.00), breast and H and N patients (P = 0.17), and pelvis and H and N patients (P = 0.18). The result also shows no significant difference between percentage deviations of measured and prescribed doses of breast, pelvis, and H and N patients (P = 0.11). Six out of the 56 patients admitted for the study have their percentage differences exceeding 5% recommended dose limit while the remaining 51 patients were within the recommended dose limits. Some researchers had mentioned that patient setup, patient movement, human errors in data transfer during the treatment procedure, patient preparation, and inaccuracies in dose calculation could cause significant higher deviation between the prescribed dose and measured dose. The response and uncertainty in the reading of the TLD detectors might also be a contributing factor.[25]
Conclusion
This study was used to determine the potential role of in vivo dosimetry as part of quality control and audit of the radiotherapy procedures in the center. There was no significant difference between values obtained and similar studies reported in the literature. This is also part of our efforts in the department and institution to conform to best radiotherapy practices as stipulated in the national and international guidelines.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- International Commission on Radiation Units and Measurements, ICRU. Prescribing, Recording and Reporting Photon Beam Therapy (Supplement to ICRU Report 50), ICRU 1999: Report 62, Bethesda, Maryland, USA; 1999.
- American Association of Physicists in Medicine AAPM. Diode in vivo Dosimetry for Patients Receiving External Beam Radiation Therapy. Report of Task Group 62 of the Radiation Therapy Committee 2005: Report 87. New York: American Institute of Physicists; 2005.
- World Health Organization (WHO). Quality Assurance in Radiotherapy. Geneva: WHO; 1988.
- International Atomic Energy Agency (IAEA). Development Procedures for in vivo Dosimetry in Radiotherapy. IAEA Human Health Reports No. 8, Vienna; 2013.
- Dam DV, Marinello G. Methods for in vivo dosimetry in external radiotherapy. In vivo dosimetry booklet. Belgium: ESTRO; 2006.
- Higgins PD, Alaei P, Gerbi BJ, Dusenbery KE. In vivo diode dosimetry for routine quality assurance in IMRT. Med Phys 2003;30:3118-23.
- Millwater CJ, MacLeod AS, Thwaites DI. In vivo semiconductor dosimetry as part of routine quality assurance. Br J Radiol 1998;71:661-8.
- Adeyemi A, Lord J. An audit of radiotherapy patient doses measured with in vivo semiconductor detectors. Br J Radiol 1997;70:399-408.
- Essers M, Mijnheer BJ. In vivo dosimetry during external photon beam radiotherapy. Int J Radiat Oncol Biol Phys 1999;43:245-59.
- Ghitulescu Z, Stochioiu A, Dumitrache M. Dose measurements in teletherapy using thermoluminescent dosimeters. Rom Rep Phys 2011;63:700-6.
- Blyth C, Macleod AS, Thwaites DS. A pilot study of the use of in vivo diode dosimetry for quality assurance in radiotherapy. Radiography 1997;3:131-42.
- Aukett RJ. A comparison of semiconductor and thermoluminescent dosemeters for in vivo dosimetry. Br J Radiol 1991;64:947-52.
- Huyskens DP, Bogaerts R, Verstraete J, Loof M, Nystrom H, Fiorino C, et al. Practical guidelines for implementation of in vivo dosimetry with diodes in external radiotherapy with photon beams (entrance dose), physics for clinical radiotherapy, ESTRO booklet 5. Brussels: ESTRO; 2001.
- Jornet N, Carrasco P, Jurado D, Ruiz A, Eudaldo T, Ribas M. Comparison study of MOSFET detectors and diodes for entrance in vivo dosimetry in 18 MV x-ray beams. Med Phys 2004;31:2534-42.
- Edwards CR, Mountford PJ. Characteristics of in vivo radiotherapy dosimetry. Br J Radiol 2009;82:881-3.
- Costa AM, Barbi GL, Bertucci EC, Ferreira H, Simone Z, Sansavino SZ, et al. In vivo dosimetry with thermoluminescent dosimeters in external photon beam radiotherapy. Appl Radiat Isot 2010;68:760-2.
- d’Angelo, Furetta C, Giaucola S, Iannoli D, Scacco AG. Verification of treatment planning systems for therapeutic irradiations using thermoluminescence dosimeters. Radiat Prot Dosimetry 1999;85:401-4.
- Leunens G, Van Dam J, Dutreix A, van der Schueren E. Quality assurance in radiotherapy by in vivo dosimetry 1. Entrance dose measurements, a reliable procedure. Radiother Oncol 1990;17:141-51.
- Noel A, Aletti P, Bey P, Malissard L. Detection of errors in individual patients in radiotherapy by systematic in vivo dosimetry. Radiother Oncol 1995;34:144-51.
- Fiorino C, Corletto D, Mangili P, Broggi S, Bonini A, Cattaneo GM, et al. Quality assurance by systematic in vivo dosimetry: Results on a large cohort of patients. Radiother Oncol 2000;56:85-95.
- Cozzi L, Fogliata-Cozzi A. Quality assurance in radiation oncology. A study of feasibility and impact on action levels of an in vivo dosimetry program during breast cancer irradiation. Radiother Oncol 1998;47:29-36.
- Aweda MA, Ibitoye AZ, Akpochafor MO, Madu BC. Entrance dose determination for selected patients at Lagos University teaching hospital, Nigeria. Radiography 2013;19:113-6.
- Ferguson HM, Lambert GD, Gustard D, Harrison RM. Tumour dose estimation using automated TLD techniques. Acta Oncol 1998;37:479-84.
- Meijer GJ, Minken AW, van Ingen KM, Smulders B, Uiterwaal H, Mijnheer BJ. Accurate in vivo dosimetry of a randomized trial of prostate cancer irradiation. Int J Radiat Oncol Biol Phys 2001;49:1409-18.
- Viegas CB, Leal MA, Viamonte A, de Araujo AC, Braz D, Clivland P. Thermoluminescent chip detector for in vivo dosimetry in H and N cancer treatment. Radiotherapy Quality Control Program of the National Cancer Institute; 2010.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.