Accelerated Brain Age in Young to Early Middle-Aged Adults after Mild to Moderate COVID-19 Infection
2 Department of Diagnostic Medicine, Dell Medical School, The University of Texas at Austin, Austin, Texas, USA
3 Department of Internal Medicine, Dell Medical School, The University of Texas at Austin, Austin, Texas, USA
4 Department of Neurology, Dell Medical School, The University of Texas at Austin, Austin, Texas, USA
Received: 27-Aug-2024, Manuscript No. amhsr-24-147879; Editor assigned: 30-Aug-2024, Pre QC No. amhsr-24-147879 (PQ); Reviewed: 13-Sep-2024 QC No. amhsr-24-147879 ; Revised: 20-Sep-2024, Manuscript No. amhsr-24-147879 (R); Published: 27-Aug-2024
Citation: Kesler SR, et al. Accelerated Brain Age in Young to Early Middle-Aged Adults after Mild to Moderate COVID-19 Infection. Ann Med Health Sci Res. 2024;14:1031-1038.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Background: Cognitive decline is a common adverse effect of the Coronavirus Disease of 2019 (COVID-19), particularly in the post-acute disease phase. The mechanisms of cognitive impairment after COVID-19 (COGVID) remain unclear, but neuroimaging studies provide evidence of brain changes, many that are associated with aging.
Aim of the study: To calculate Brain Age Gap (BAG), which is the difference between brain age and chronological age.
Material and Methods: BAG was measured in a cohort of 25 mild to moderate COVID-19 survivors (did not experience breathlessness, pneumonia, or respiratory/organ failure) and 24 non-infected controls (mean age=30 ± 8) using Magnetic Resonance Imaging (MRI).
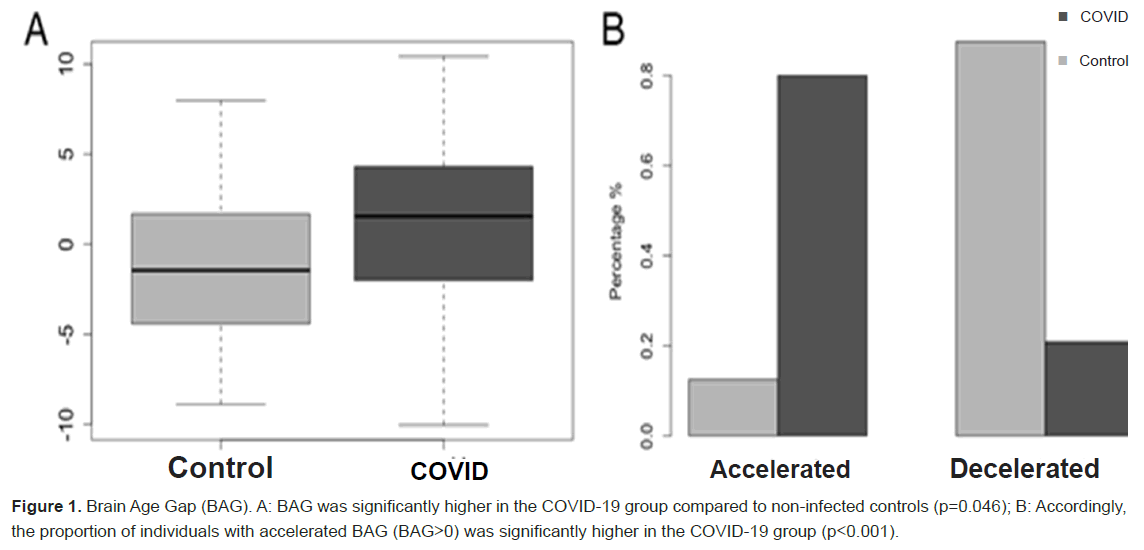
Results: BAG was significantly higher in the COVID-19 group (F=4.22, p=0.046) by 2.65 years. Additionally, 80% of the COVID-19 group demonstrated an accelerated BAG compared to 13% in the control group (X2=20.0, p<0.001). Accelerated BAG was significantly correlated with lower cognitive function (p<0.041). Females in the COVID-19 group demonstrated a 99% decreased risk of accelerated BAG compared to males (OR=0.015, 95% CI: 0.001 to 0.300). There was also a small (1.4%) but significant decrease in risk for accelerated BAG associated with longer time since COVID-19 diagnosis (OR=0.986, 95% CI: 0.977 to 0.995).
Conclusion: Our findings provide a novel biomarker of COGVID and point to accelerated brain aging as a potential mechanism of this adverse effect. Our results also offer further insight regarding gender-related disparities in cognitive morbidity associated with COVID-19.
Keywords
COVID-19; Brain age; Cognition; MRI (Magnetic Resonance Imaging); Angiotensin- Converting Enzyme 2 (ACE2)
Introduction
Emerging studies have demonstrated that the Coronavirus Disease of 2019 (COVID-19) increase the risk for a range of neurological difficulties including cognitive impairment. Cognitive decline, or “brain fog”, is believed to be part of a cluster of symptoms that encompass difficulty with concentration, attention and memory that persist beyond the acute disease phase. These symptoms are known as Post-Acute Sequelae of SARS-CoV-2 Infection (PASC) or Long COVID [1]. Cognitive impairment after COVID-19 (COGVID) reduces quality of life, extends disease related morbidity and impairs ability to return to work [2,3]. The precise mechanism of COGVID is unclear though neuroimaging studies have demonstrated significant alterations in brain structure and function [4-13], including studies of noncritical/ non-hospitalized cases [14-17].
We previously showed that survivors of mild to moderate, nonhospitalized COVID-19 have significantly lower functional brain connectivity compared to non-infected controls and hypoconnectivity was correlated with lower cognitive performance [18]. Prior studies have indicated that reduction in functional brain connectivity is associated with normal aging [19,20]. Given that our cohort of COVID-19 survivors was young (mean age=30 years), accelerated brain aging may be a potential mechanism of cognitive impairment in this population. Prior studies of COGVID have focused on older adults with severe disease as these are the most vulnerable patients. However, this approach has created a gap in the literature regarding cognitive outcomes in survivors of mild to moderate COVID-19 (people that do not experience breathlessness, pneumonia or respiratory/organ failure), as younger individuals also show significant COGVID and may in fact be more vulnerable to long-term impairment [18].
Brain age can be estimated using non-invasive neuroimaging in combination with artificial intelligence. Comparing estimated brain age with chronological age provides a metric known as Brain Age Gap (BAG), which quantifies brain health as the divergence from the typical trajectory of aging. Multiple studies have shown that BAG is a sensitive biomarker for detecting various neurologic and neuropsychiatric conditions [21-26]. We aimed to examine BAG in young and early middle-aged adults who had mild to moderate COVID-19. We hypothesized that BAG would be higher in COVID-19 survivors compared to non-infected controls.
Materials and Methods
Participants
Between October, 2021 and January, 2023 we recruited adults with and without history of COVID-19 in central Texas. Potential participants were recruited via social media, community boards and outpatient referrals. Participants in the COVID-19 group were excluded for signs or symptoms of severe infection including self-rating of severity or hospitalization. Any potential participant was excluded for pre-existing history of developmental, medical, or psychiatric disorders known to affect cognitive function, significant sensory impairment (e.g., blindness), or MRI contraindications (e.g., magnetic biomedical implants, certain orthodontia, claustrophobia). In total, we enrolled 50 adults (54% female) aged 21 to 50 years (mean=30.7 ± 8.7). Twenty-six participants had a self-reported history of positive COVID-19 test and the remaining 24 participants self-reported no history of infection by test or associated symptoms. We excluded one participant in the COVID-19 group for receiving treatment in a Post-COVID Clinic for Long COVID. There were no significant differences between the two groups in any demographic characteristics except for racial/ethnic minority status which was significantly higher in the control group (Table 1). This study was approved by the University of Texas at Austin Institutional Review Board (protocol #00001337), was conducted in accordance with the declaration of Helsinki and all participants provided written, informed consent.
| COVID N=25 | Control N=24 | stat | p-value | |
|---|---|---|---|---|
| Age (years) | 30.3 (8.0) | 30.3 (9.0) | t=-0.003 | 0.997 |
| Education (years) | 16.4 (1.8) | 16.9 (1.6) | t=0.995 | 0.325 |
| Female gender | N=14 (56.0%) | N=13 (54.2%) | X2=0.017 | 0.897 |
| Income<$100K | N=12 (48%) | N=8 (33.3%) | X2=1.113 | 0.292 |
| Racial/ethnic minority | N=3 (12.0%) | N=11 (45.8%) | X2=6.868 | 0.009 |
| Time since diagnosis (days) | 307 (223) | _ | _ | _ |
| Range: 12-719 | _ | _ | _ | |
| COVID severity moderate | N=11 (44.0%) | _ | _ | _ |
| No. of symptoms during active COVID infection | Median=8.0, IQR=4.6 | _ | _ | _ |
| Range=1-16 out of 20 possible | _ | _ | _ |
Table 1: Demographic and COVID-19 Characteristics. Data are shown as mean (standard deviation) unless otherwise indicated.
Demographic and COVID-19 measures
We used instruments recommended by the National Institutes of Health to facilitate COVID-19-related research. Specifically, from the NIH Repository of COVID-19 Research Tools, we administered the RADxUP Sociodemographic Questionnaire and the COVID-19 Experiences questionnaire to measure COVID-19 severity and symptoms during active infection [27]. We also assessed current anosmia using the pocket smell test [28].
Neuropsychiatric function
We administered braincheck, a computerized battery of neuropsychological tests including trails A (attention, processing speed, executive function), trails B (attention, processing speed, executive function), immediate list recall (verbal memory learning), Delayed list recall (verbal memory recall), Stroop (response inhibition) and digit symbol (graphomotor processing speed) [29]. BrainCheck provides age normalized test scores (mean=100 ± 15) and has shown adequate discriminant validity in COVID-19 and non-COVID-19 populations [30].
To measure subjective cognitive function, we administered the Patient Reported Outcome Measures Information System (PROMIS) Cognitive Function Short Form 8a [31]. This is an 8-item, self-rating questionnaire regarding the frequency of cognitive symptoms which has shown high internal consistency reliability (Cronbach’s α>0.89) and adequate convergent validity (r>|0.77|) Iverson, 2021 #10658;Valentine, 2019 #10660. We also administered Patient-Reporting Outcomes Measurement Information System (PROMIS-57) Profile v2.1 to measure depressive symptoms, fatigue, anxiety, sleep disturbance, pain and social role functioning all of which have shown strong internal consistency reliability (Cronbach’s α>0.84) and moderate to strong convergent validity (r>|0.57|) [32]. PROMIS provides standardized T-scores with a mean of 50 and standard deviation of 10.
Neuroimaging pulse sequence
T1-weighted anatomical MRI was collected using a highresolution Magnetization Prepared Rapid Gradient Echo (MPRAGE) sequence: TR=2400 ms, TE=2.18, flip angle=8 degrees, FOV 256 mm, parallel imaging with GRAPPA (Generalized Auto-calibrating Partial Parallel Acquisition) acceleration factor=2, 0.8 mm isotropic resolution, 208 sagittal slices, scan time=6:38 min. Resting state functional Magnetic Resonance Imaging (fMRI), diffusion tensor imaging and arterial spin labeling data were also collected during this 45 min scan session but are not reported here.
Brain Age Gap (BAG)
We estimated brain age from brain volumes with BrainAgeR v2.1, a publicly available algorithm that has been shown to be one of the most reliable for predicting age from brain MRI [33]. The algorithm implements a Gaussian Processes regression model to predict brain age from segmented brain volumes [34,35]. The model was trained on 3,377 healthy individuals (mean age=40.6 years, SD=21.4, age range 18-92 years) and tested on an independent dataset of 857 healthy individuals (mean age=40.1 years, SD=21.8, age range 18-90 years). The algorithm accepts raw, T1-weighted MRI scans, segments and normalizes them using Statistical Parametric Mapping v12 (Wellcome Trust Centre for Neuroimaging, London, UK) with custom templates. We subtracted chronological age from estimated brain age to calculate BAG. A positive BAG thus represents accelerated brain age and a negative BAG represents decelerated brain age [23].
Statistical analysis
We compared BAG between groups using two different approaches; Analysis Of Covariance (ANCOVA) controlling for total brain volume and a Chi square test to evaluate the difference in frequency of accelerated BAG (BAG>0). We conducted exploratory Spearman correlation analysis between accelerated BAG (1=yes, 0=no) and cognitive test scores. We used exploratory logistic regression to determine clinical and demographic characteristics (number of days since COVID-19 diagnosis, number of COVID-19 symptoms, sex, COVID-19 severity and racial/ethnic minority classification) associated with accelerated BAG. Cognitive tests were compared using t-tests for continuous variables and Chi Squared tests for categorical variables during our previously study of this cohort with False Discovery Rate (FDR) correction for multiple comparisons [18]. Alpha level for all tests was set at p<0.05 and all data visualizations and analyses were conducted in the R Statistical Package v4.3.1.
Results
COVID-19 group characteristics
As we previously reported for this group, participants with a history of COVID-19 infection were nearly evenly split between mild and moderate disease severity with relatively low symptom burden during the acute infection [18]. There was a wide range in the interval between COVID-19 diagnosis and study enrollment (12-719 days, mean of 10 ± 7 months) (Table 1). No participants demonstrated anosmia (Table 2).
| COVID N=25 | Control N=24 | Stat (W/X2) | Uncorrected p-value | FDR corrected p-value | Rank biserial correlation | |
|---|---|---|---|---|---|---|
| Trails A | 97.2 (11.9) | 104.4 (13.7) | 372.5 | 0.081 | 0.211 | 0.296 |
| Trails B | 98.6 (11.9) | 100.8 (15.5) | 333 | 0.353 | 0.386 | 0.158 |
| Digit symbol substitution | 95.0 (14.8) | 99.2 (15.1) | 353.5 | 0.289 | 0.386 | 0.178 |
| Stroop | 94.4 (19.4) | 101.1 (15.8) | 341.5 | 0.269 | 0.386 | 0.188 |
| Immediate recall | 100.2 (18.9) | 106.5 (9.8) | 344 | 0.356 | 0.386 | 0.147 |
| Delayed recall | 102.4 (13.8) | 106.5 (10.5) | 359.5 | 0.219 | 0.386 | 0.198 |
| PROMIS cognitive | 49.4 (11.4) | 60.1 (6.2) | 475 | 0 | <0.001 | 0.583 |
| Impaired PROMIS cognitive (T score<55) | 18 (72%) | 6 (25%) | 10.8 | 0.001 | 0.007 | - |
| PROMIS social role performance | 57.2 (9.7) | 59.1 (8.4) | 332 | 0.494 | 0.494 | 0.107 |
| PROMIS anxiety | 55.0 (11.6) | 48.0 (9.4) | 195 | 0.035 | 0.199 | -0.35 |
| PROMIS depression | 48.9 (8.9) | 43.7 (5.4) | 202.5 | 0.046 | 0.199 | -0.325 |
| PROMIS fatigue | 49.9 (9.9) | 44.4 (9.2) | 212 | 0.078 | 0.211 | -0.293 |
| PROMIS sleep disturbance | 50.9 (9.8) | 46.5 (7.2) | 236 | 0.202 | 0.386 | -0.213 |
| PROMIS pain | 45.5 (7.1) | 43.8 (6.0) | 260 | 0.333 | 0.386 | -0.133 |
| Anosmia (Pocket Smell Test score<2) | 0 (0%) | 0 (0%) | - | - | - | - |
Table 2: Neuropsychiatric testing performance. Note: Data are shown as mean (standard deviation); Rank biserial correlation is the effect size; FDR=False Discovery Rate correction for multiple comparisons.
Neuropsychiatric function
The COVID-19 group also demonstrated lower performance on all cognitive tests (effect size r=0.15 to 0.30) [18]. However, only PROMIS cognitive score was significantly different between groups (p<0.001, FDR corrected, effect size r=0.58) and the COVID-19 group demonstrated significantly higher incidence of PROMIS Cognitive impairment (X2=10.8, p=0.007, FDR corrected). The COVID-19 group also endorsed greater symptoms of anxiety and depression; however, these were not significantly different between groups after correction for multiple comparisons (Table 2).
Between group difference in BAG. As shown in Figure 1, mean BAG was significantly higher in the COVID-19 group (F=4.22, p=0.046) by 2.65 years. Additionally, there were N=20 (80%) in the COVID-19 group who demonstrated an accelerated BAG compared to N=3 (13%) in the control group, which was a significant difference (X2=20.0, p<0.001).
Relationship between accelerated BAG and cognitive function
Clinical and demographic characteristics of accelerated BAG
The overall logistic regression model was significant (X2=14.71, Nagelkerke R2=0.666, p=0.012) (Table 3). An increased duration from COVID-19 diagnosis was associated with a small yet statistically significant decrease (1.4%) in the risk of accelerated BAG. There was also a large (99%) and significant decreased risk of accelerated BAG in females compared to males with COVID-19.
| Odds Ratio | 95% CI lower | 95% CI upper | |
|---|---|---|---|
| Time since COVID diagnosis (days) | 0.986 | 0.977 | 0.995 |
| Number of COVID symptoms* | 1.35 | 0.873 | 2.09 |
| Female biological sex | 0.015 | 0.001 | 0.3 |
| Moderate COVID severity | 6.82 | 0.088 | 526 |
| Racial/ethnic minority | 31.52 | 1.55 | 642 |
Table 3: Neuropsychiatric Testing Performance. Note: Data are shown as mean (standard deviation). Rank biserial correlation is the effect size; FDR=False Discovery Rate correction for multiple comparisons; * During acute infection
There were N=9/14 females (64%) in the COVID-19 group with accelerated BAG compared to 10/11 males (91%). Increased number of symptoms during acute infection, moderate COVID-19 severity and racial/ethnic minority status were associated with increased risk for accelerated BAG but were not statistically significant and lacked precision in this small sample (i.e., large confidence intervals for odds ratios).
Discussion
Cognitive impairment after COVID-19 (COGVID) appears to be one the most common long-term side effects of infection yet the mechanisms of this problem are an area of active research. In this study, we identified accelerated brain aging as a potential contributor to COGVID [1]. Individuals with a history of COVID-19 infection demonstrated a 2.65 year accelerated BAG compared to the control group. We also found that poorer performance on an objective measure of attention, processing speed and executive function (Trails A), as well as greater subjective cognitive problems were associated with higher BAG. Finally, we identified a significantly higher risk of accelerated BAG among males compared to females with mild to moderate COVID-19 infection.
COVID-19 induced upregulation of Angiotensin-Converting Enzyme 2 (ACE2) expression in the brain is a potential COVID-19 induced upregulation of Angiotensin-Converting Enzyme 2 (ACE2) expression in the brain is a potential mechanism for our finding of accelerated BAG. SARS-CoV-2, the virus responsible for COVID-19, uses ACE2 receptors to enter human cells [36]. Furthermore, ACE2 expression is upregulated in the brain after COVID-19, especially in patients with significant neurologic symptoms [37]. ACE2 is primarily involved in the Renin-Angiotensin System (RAS). While the RAS is primarily known for its role in regulating blood pressure and fluid balance, it is also crucial for brain health and cognitive function [38]. The RAS system becomes dysregulated with increasing age, contributing to the pathogenesis of neurodegenerative diseases [39,40]. The specific role of ACE2 in neurodegeneration is complex given that its expression is associated with neuroprotective effects, yet several studies have noted elevated ACE2 levels in patients with alzheimer’s disease [41-43]. These studies suggest that chronic elevation of ACE2 may result in dysregulation of the RAS system, increasing the risk for accelerated brain aging. To further evaluate this hypothesis, future studies should assess ACE2 level in Long COVID patients with cognitive impairment.
Alternatively, the impact of ACE2 expression may vary depending on its distribution across different brain regions [44]. Some areas might be more sensitive to changes in ACE2 levels, leading to localized neurodegeneration despite the overall neuroprotective effects of ACE2. Our group and others have shown that prefrontal cortex is preferentially susceptible to COVID-19 [9,14,15,18]. Under normal conditions, ACE2 expression is extremely low in prefrontal cortex and thus this region may be exceptionally vulnerable to ACE2 elevation [45]. Currently available brain age algorithms do not provide regional BAGs, but these could be estimated using custom models in large samples.
COGVID may also result from accelerated aging due in part to chronic inflammation, or “inflammaging” [46,47]. Inflammation is well-known to play a critical role in neurodegeneration [48,49]. Patients with COVID-19 demonstrate post-infection upregulation of inflammatory biomarkers with the extent of the inflammatory response depending on disease severity [50,51]. Research among middle-aged COVID-19 survivors has shown significant inflammation-related astrocytic damage and neural dysfunction regardless of disease severity [52]. Inflammation after COVID-19 infection may also impair cognition via reduced serotonin levels [53]. The prefrontal cortex is particularly vulnerable to inflammation due to its unique reliance on glutamate receptor/calcium mediated neurotransmission [54]. The relationship between inflammation and BAG over time in mild and moderate COVID-19 survivors requires further study.
BAG is associated with certain genetic as well as lifestyle factors and may therefore be useful in treatment monitoring and development [23,55,56]. Dietary and nutritional interventions have been effective in reducing brain aging, particularly calorie restriction and low consumption of processed food and sweets [57,58]. Physical activity has consistently been associated with improved cognitive function and decreased brain aging [59,60]. However, using physical activity to treat long COVID is currently highly controversial in clinical practice because of the risk for inducing post-exertional malaise. There appears to be a complex interaction between exercise and immune-mediated symptoms, especially among younger COVID-19 survivors and therefore, physical activity may not be the best recommendation for many patients [50]. Current guidance on Long COVID emphasizes that physical activity should be individualized and structured, titrated carefully to avoid post-exertional malaise [61]. However, other modifiable factors associated with brain age include sleep disruption, anxiety and depression [60]. Interventions that address these symptoms, such as cognitive behavioral therapy may protect against accelerated brain aging [62,63]. Future research is necessary to elucidate the protective mechanisms of healthy lifestyle habits on brain health and aging among patients with COVID-19.
COVID-19 mortality and morbidity are higher in men compared to women [64]. Some studies have observed sex differences in COGVID while others have not [65-67]. We found that males scored more poorly than females on a measure of attention and processing speed during a prior case series study involving a different sample at approximately 4 months post-infection [68]. In the present group, at approximately 10 months post-infection, we found no sex differences in cognitive outcomes [18] but males had a 99% greater risk of accelerated BAG. The mechanisms of this sex difference remain unclear but may involve ACE2 pathways [69]. For example, Swärd et al., showed that peripheral ACE2 expression is higher in males and increases more with age compared to females, at least in early adulthood [70]. It is unknown if there is greater cerebral ACE2 upregulation in males, but it is plausible given the peripheral difference and would potentially support ACE2 targeted therapies for this population.
We also found that there was a statistically significant decrease in risk of accelerated BAG associated with longer time since COVID-19 diagnosis. Given that this was only 1.4%, it may not be clinically meaningful. Alternatively, this finding may point to one or more subgroups of patients whose brain health significantly improves over time. If so, BAG may be useful in larger samples for biotyping (biologically stratifying) patients with different trajectories of brain health after COVID-19. Thus, BAG biotypes could improve precision medicine by identifying subgroups of patients at highest risk for COGVID [71]. Evidence shows that a significant number of patients with post-COVID symptoms have considerable symptom resolution within a year after infection [72-74]. Thus, longitudinal studies of COVID-19 survivors are needed to determine if BAG also improves over time and if so, in which patients.
Strengths of our study include the focus on mild to moderate COVID-19, whereas prior neuroimaging research has focused on post-hospitalization patients, the emphasis on younger survivors who may be more vulnerable to long-term cognitive effects, the novel examination of brain age in this population, the use of a well-established algorithm for estimating brain age, the inclusion of a control group and the use of both objective and subjective cognitive assessments. Weaknesses include small sample, cross-sectional design which precludes insight regarding how participants functioned cognitively prior to COVID-19 and what individual cognitive trajectories may exist, self-reported nature of COVID diagnosis/symptoms and the lack of data regarding lifestyle factors associated with brain aging (e.g., diet, smoking status) and other post-acute symptoms such as post-exertional malaise, ageusia, chronic cough, etc. that could be used to determine if any participants had other characteristics of Long COVID. There are several alternative algorithms available for estimating brain age that may yield different results, although we chose the one with the highest reliability based on the existing literature. Additionally, the brainageR algorithm utilizes gray, white and CSF volumes whereas other algorithms use only gray matter. However, BAGs derived from different imaging modalities may represent different endophenotypes and thus multimodal BAGs may be informative within larger, future COVID-19 studies [23].
Conclusion
In summary, our findings support our hypothesis that brain age is accelerated in survivors of COVID-19 infection compared to non-infected controls. This study represents the first examination of this innovative biomarker in post-COVID-19 neurocognitive dysfunction. Whereas samples from previous works have primarily included older adults, our study focused on a younger group of individuals to examine their specific cognitive vulnerabilities and associated neural mechanisms. Our cohort, observed on average after a longer duration post- COVID-19 infection than those in previous studies, offers distinct insights into the protracted effects of COVID-19 on brain health. Additionally, our findings contribute significant new information regarding the disparity in outcomes for men post-COVID-19.
Acknowledgement
The authors would like to thank the faculty of the Biomedical Imaging Center at The University of Texas at Austin.
Previous Presentation of the Study
A preprint of this manuscript has previously been published (Kesler SR, Franco-Rocha OY, De La Torre Schutz A, et al.; Accelerated brain age in young to early middle-aged adults after mild to moderate COVID-19 infection. medRxiv preprint 2024. doi: 10.1101/2024.03.05.24303816.)
Criteria for Inclusion in the Authors’ List
To be included as an author, the person had to substantially contribute to the study's conception, design, data acquisition, analysis, or interpretation, as well as have participated in drafting or revising the manuscript.
Authors Declaration of Approval
The manuscript has been read and approved by all the authors, the requirements for authorship as stated earlier in this document have been metand each author believes that the manuscript represents honest work.
Data Sharing
The datasets generated and analyzed during the current study are not publicly available due the fact that they constitute an excerpt of research in progress but will be available on the EBRAINS platform (https://www.ebrains.eu) under the corresponding author’s name at study completion. Interested parties may contact the corresponding author for further information.
References
- Thaweethai T, Jolley SE, Karlson EW, Al-Aly Z, Diaz JV, et al. Development of a definition of postacute sequelae of SARS-CoV-2 infection. JAMA. 2023; 329:1934-1946.
[Google Scholar] [PubMed]
- Perlis RH, Lunz Trujillo K, Safarpour A, Green J, Santillana M, et al. Association of post-COVID-19 condition symptoms and employment status. JAMA Netw Open. 2023;6:e2256152.
[Crossref] [Google Scholar] [PubMed]
- Han JH, Womack KN, Tenforde MW, Halasa NB, Edwards KM, et al. Associations between persistent symptoms after mild COVID-19 and long-term health status, quality of life, and psychological distress. Influenza Other Respir Viruses. 2022; 16:680-689.
[Crossref] [Google Scholar] [PubMed]
- Niroumand Sarvandani M, Sheikhi Koohsar J, Rafaiee R, Shirvani N, Golbabaie A, et al. COVID-19 and the Brain: A Psychological and Resting-state fMRI Study of the Whole-brain Functional Connectivity. Basic Clin Neurosci. 2021; bcn.2021.1425.4.
[PubMed]
- Duan K, Premi E, Pilotto A, Alberici E, Gasparotti R, et al. Alterations of frontal-temporal gray matter volume associate with clinical measures of older adults with COVID-19. Neurobiol Stress. 2021;14:100326.
[Crossref] [Google Scholar] [PubMed]
- Huang S, Zhou X, Zhao W, Wang H, Meng Y, et al. Dynamic white matter changes in recovered COVID-19 patients: A two-year follow-up study. Theranostics. 2023; 13:724-735.
[Crossref] [Google Scholar] [PubMed]
- Voruz P, Cionca A, Jacot de Alcantara I, Allali G, Pignatti R, et al. Functional connectivity underlying cognitive and psychiatric symptoms in post-COVID-19 syndrome: Is anosognosia a key determinant? Brain Commun. 2022; 4:fcac057.
[Crossref] [Google Scholar] [PubMed]
- Tassignon B, Radwan A, Blommaert J, Volckaert T, Maes J, et al. Longitudinal changes in global structural brain connectivity and cognitive performance in former hospitalized COVID-19 survivors: An exploratory study. Exp Brain Res. 2023; 241:727-741.
[Crossref] [Google Scholar] [PubMed]
- Paolini M, Palladini M, Mazza MG, Valenti M, Rovere-Querini P, et al. Brain correlates of subjective cognitive complaints in COVID-19 survivors: A multimodal magnetic resonance imaging study. Eur Neuropsychopharmacol. 2023; 68:1-10.
[Crossref] [Google Scholar] [PubMed]
- Voruz P, Cionca A, Jacot de Alcantara I, Assal F, Allali G, et al. Brain functional connectivity alterations associated with neuropsychological performance 6-9 months following SARS-CoV-2 infection. Hum Brain Mapp. 2023; 44:1629-1646.
[Crossref] [Google Scholar] [PubMed]
- Diez-Cirarda M, Yus M, Gomez-Ruiz N, Ojeda N, Peña J, et al. Multimodal neuroimaging in post-COVID syndrome and correlation with cognition. Brain. 2023; 146:2142-2152.
[Crossref] [Google Scholar] [PubMed]
- Tian T, Wu J, Chen T, Zhao B, Chen Y, et al. Long-term follow-up of dynamic brain changes in patients recovered from COVID-19 without neurological manifestations. JCI Insight. 2022;7:e155827.
[Crossref] [Google Scholar] [PubMed]
- Lu Y, Li X, Geng D, Mei N, Wu PY, et al. Cerebral Micro-Structural Changes in COVID-19 Patients-An MRI-based 3-month Follow-up Study. EClinicalMedicine. 2020; 25:100484.
[Crossref] [Google Scholar] [PubMed]
- Douaud G, Lee S, Alfaro-Almagro F, Arthofer C, Wang C, et al. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature. 2022; 604:697-707.
[Crossref] [Google Scholar] [PubMed]
- Ajcevic M, Iscra K, Furlanis G, Marsic T, Basaldella F, et al. Cerebral hypoperfusion in post-COVID-19 cognitively impaired subjects revealed by arterial spin labeling MRI. Sci Rep. 2023;13:5808.
[Crossref] [Google Scholar] [PubMed]
- Gulyaev SA. EEG microstate analysis and the EEG inverse problem solution as a tool for diagnosing cognitive dysfunctions in individuals who have had a mild form of COVID-19. Hum Physiol. 2022; 48:587-597.
- Silva LS, Ludwig GV, Rocha C, Souza TK, da Costa BA, et al. Functional and microstructural brain abnormalities, fatigue, and cognitive dysfunction after mild COVID-19. Medrxiv. 2021; 2021-03.
[Crossref] [Google Scholar] [PubMed]
- Kesler SR, Rocha OY, Schutz AD, Lewis KA, Brode M, et al. Altered functional brain connectivity, efficiency, and information flow associated with brain fog after mild to moderate COVID-19 infection. 2023.
- Ferreira LK, Regina AC, Amaro Jr E, McIntosh AR, Busatto GF, et al. Aging effects on whole-brain functional connectivity in adults free of cognitive and psychiatric disorders. Cerebral cortex. 2016; 26:3851-3865.
[Crossref] [Google Scholar] [PubMed]
- Varangis E, Habeck CG, Razlighi QR, Stern Y. The effect of aging on resting state connectivity of predefined networks in the brain. Frontiers in aging neuroscience. 2019; 11:234.
- Danesh V, Arroliga AC, Bourgeois JA, Boehm LM, Kesler SR, et al. Symptom clusters seen in adult COVID-19 recovery clinic care seekers. Journal of general internal medicine. 2023; 38:442-449.
[Crossref] [Google Scholar] [PubMed]
- Altuna M, Sánchez-Saudinós MB, Lleó A. Cognitive symptoms after COVID-19. Neurology perspectives. 2021; 1:16-24.
[Crossref] [Google Scholar] [PubMed]
- Wen J, Zhao B, Yang Z, Erus G, Boquet-Pujadas A, et al. The Genetic Heterogeneity of Multimodal Human Brain Age. bioRxiv. 2023; 15:2023-04.
[Crossref] [Google Scholar] [PubMed]
- Cole JH, Marioni RE, Harris SE, Deary IJ. Brain age and other bodily ‘ages’: Implications for neuropsychiatry. Molecular psychiatry. 2019; 24:266-81.
[Crossref] [Google Scholar] [PubMed]
- Mishra S, Beheshti I, Khanna P. A review of neuroimaging-driven brain age estimation for identification of brain disorders and health conditions. IEEE Reviews in Biomedical Engineering. 2021; 16:371-85.
- Ballester PL, Romano MT, de Azevedo Cardoso T, Hassel S, Frey BN, et al. Brain age in mood and psychotic disorders: A systematic review and meta-analysis. Acta Psychiatrica Scandinavica. 2022; 145:42-55.
[Crossref] [Google Scholar] [PubMed]
- Pan H, Tryka KA, Junkins HA, Ramos EM, Hamilton CM, et al. Using PhenX measures to identify opportunities for cross-study analysis. Human mutation. 2012; 33:849-57.
[Crossref] [Google Scholar] [PubMed]
- Duff K, McCaffrey RJ, Solomon GS. The pocket smell test: Successfully discriminating probable Alzheimer's dementia from vascular dementia and major depression. The Journal of neuropsychiatry and clinical neurosciences. 2002;14:197-201.
[Crossref] [Google Scholar] [PubMed]
- Groppell S, Soto-Ruiz KM, Flores B, Dawkins W, Smith I, et al. A rapid, mobile neurocognitive screening test to aid in identifying cognitive impairment and dementia (BrainCheck): Cohort study. JMIR aging. 2019; 2:12615.
[Crossref] [Google Scholar] [PubMed]
- Franco-Rocha OY, Mahaffey ML, Matsui W, Kesler SR. Remote assessment of cognitive dysfunction in hematologic malignancies using web-based neuropsychological testing. Cancer Medicine. 2023; 12:6068-76.
- Jensen RE, Potosky AL, Moinpour CM, Lobo T, Cella D, et al. United States population-based estimates of patient-reported outcomes measurement information system symptom and functional status reference values for individuals with cancer. Journal of Clinical Oncology. 2017;35:1913-20.
[Crossref] [Google Scholar] [PubMed]
- Cella D, Riley W, Stone A, Rothrock N, Reeve B, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. Journal of clinical epidemiology. 2010;63:1179-94.
[Crossref] [Google Scholar] [PubMed]
- Bacas E, Kahhalé I, Raamana PR, Pablo JB, Anand AS, et al. Probing multiple algorithms to calculate brain age: Examining reliability, relations with demographics, and predictive power. Human Brain Mapping. 2023;44:3481-92.
[Crossref] [Google Scholar] [PubMed]
- Ole J. brainageR. 2023.
- Cole JH, Poudel RP, Tsagkrasoulis D, Caan MW, Steves C, et al. Predicting brain age with deep learning from raw imaging data results in a reliable and heritable biomarker. NeuroImage. 2017;163:115-24.
[Crossref] [Google Scholar] [PubMed]
- Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, et al. SARS-CoV-2 cell entry depends on ACE2, TMPRSS2 and is blocked by a clinically proven protease inhibitor. cell. 2020;181:271-80.
[Crossref] [Google Scholar] [PubMed]
- Lindskog C, Méar L, Virhammar J, Fa¨llmar D, Kumlien E, et al. Protein expression profile of ACE2 in the normal and COVID-19-affected human brain. Journal of Proteome Research. 2022;21:2137-45.
[Crossref] [Google Scholar] [PubMed]
- Ghalayini J, Boulianne GL. Deciphering mechanisms of action of ACE inhibitors in neurodegeneration using Drosophila models of Alzheimer’s disease. Frontiers in Neuroscience. 2023 Apr 11;17:1166973.
[Crossref] [Google Scholar] [PubMed]
- Conti S, Cassis P, Benigni A. Aging and the renin-angiotensin system. Hypertension. 2012;60:878-83.
- Cosarderelioglu C, Nidadavolu LS, George CJ, Oh ES, Bennett DA, et al. Brain renin–angiotensin system at the intersect of physical and cognitive frailty. Frontiers in neuroscience. 2020;14:586314.
[Crossref] [Google Scholar] [PubMed]
- Reveret L, Leclerc M, Emond V, Tremblay C, Loiselle A, et al. Higher angiotensin-converting enzyme 2 (ACE2) levels in the brain of individuals with Alzheimer’s disease. Acta neuropathologica communications. 2023;11:159.
[Crossref] [Google Scholar] [PubMed]
- Ding Q, Shults NV, Gychka SG, Harris BT, Suzuki YJ. Protein expression of angiotensin-converting enzyme 2 (ACE2) is upregulated in brains with Alzheimer’s disease. International journal of molecular sciences. 2021;22:1687.
[Crossref] [Google Scholar] [PubMed]
- Zhao Y, Li W, Lukiw W. Ubiquity of the SARS-CoV-2 receptor ACE2 and upregulation in limbic regions of Alzheimer’s disease brain. Folia neuropathologica. 2021;59:232-8.
[Crossref] [Google Scholar] [PubMed]
- Cui H, Su S, Cao Y, Ma C, Qiu W. The altered anatomical distribution of ACE2 in the brain with Alzheimer’s disease pathology. Frontiers in Cell and Developmental Biology. 2021;9:684874.
[Crossref] [Google Scholar] [PubMed]
- Chen R, Wang K, Yu J, Howard D, French L, et al. The spatial and cell-type distribution of SARS-CoV-2 receptor ACE2 in the human and mouse brains. Frontiers in neurology. 2021;11:573095.
[Crossref] [Google Scholar] [PubMed]
- Bektas A, Schurman SH, Franceschi C, Ferrucci L. A public health perspective of aging: Do hyper-inflammatory syndromes such as COVID-19, SARS, ARDS, cytokine storm syndrome, and post-ICU syndrome accelerate short-and long-term inflammaging?. Immunity & Ageing. 2020;17:1-0.
[Crossref] [Google Scholar] [PubMed]
- Müller-Werdan U, Polidori MC, Simm A. On frailty and accelerated aging during SARS-Cov-2: Senescence. Aging Clinical and Experimental Research. 2023;35:907-12.
[Crossref] [Google Scholar] [PubMed]
- Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918-34.
[Crossref] [Google Scholar] [PubMed]
- Walker KA, Ficek BN, Westbrook R. Understanding the role of systemic inflammation in Alzheimer’s disease. ACS chemical neuroscience. 2019;10:3340-2.
[Crossref] [Google Scholar] [PubMed]
- Silva BS, Pereira T, Minuzzi LG, Padilha CS, Figueiredo C, et al. Mild to moderate post-COVID-19 alters markers of lymphocyte activation, exhaustion, and immunometabolic responses that can be partially associated by physical activity level-an observational sub-analysis fit-COVID study. Frontiers in Immunology. 2023;14:1212745.
[Crossref] [Google Scholar] [PubMed]
- Soares-Schanoski A, Sauerwald N, Goforth CW, Periasamy S, Weir DL, et al. Asymptomatic SARS-CoV-2 infection is associated with higher levels of serum IL-17C, matrix metalloproteinase 10 and fibroblast growth factors than mild symptomatic COVID-19. Frontiers in Immunology.2022; 13:821730.
[Crossref] [Google Scholar] [PubMed]
- Passos FR, Heimfarth L, Monteiro BS, Correa CB, de Moura TR, et al. Oxidative stress and inflammatory markers in patients with COVID-19: Potential role of RAGE, HMGB1, GFAP and COX-2 in disease severity. International Immunopharmacology. 2022;104:108502.
[Crossref] [Google Scholar] [PubMed]
- Wong AC, Devason AS, Umana IC, Cox TO, Dohnalová L, et al. Serotonin reduction in post-acute sequelae of viral infection. Cell. 2023;186:4851-67.
[Crossref] [Google Scholar] [PubMed]
- Arnsten AF, Datta D, Wang M. The genie in the bottle-magnified calcium signaling in dorsolateral prefrontal cortex. Molecular Psychiatry. 2021;26:3684-700.
[Crossref] [Google Scholar] [PubMed]
- Wrigglesworth J, Ward P, Harding IH, Nilaweera D, Wu Z, et al. Factors associated with brain ageing-a systematic review. BMC neurology. 2021;21:312.
[Crossref] [Google Scholar] [PubMed]
- Kaufmann T, van der Meer D, Doan NT, Schwarz E, Lund MJ, et al. Common brain disorders are associated with heritable patterns of apparent aging of the brain. Nature neuroscience. 2019;22:1617-23.
[Crossref] [Google Scholar] [PubMed]
- Trajano GS, Blazevich AJ. Static stretching reduces motoneuron excitability: The potential role of neuromodulation. Exercise and sport sciences reviews. 2021;49:126-32.
[Crossref] [Google Scholar] [PubMed]
- Levakov G, Kaplan A, Meir AY, Rinott E, Tsaban G, et al. The effect of weight loss following 18 months of lifestyle intervention on brain age assessed with resting-state functional connectivity. ELife. 2023;12:e83604.
[Crossref] [Google Scholar] [PubMed]
- Stillman CM, Esteban-Cornejo I, Brown B, Bender CM, Erickson KI. Effects of exercise on brain and cognition across age groups and health states. Trends in neurosciences. 2020;43:533-43.
[Crossref] [Google Scholar] [PubMed]
- Chen ST, Volle D, Jalil J, Wu P, Small GW. Health-promoting strategies for the aging brain. The American Journal of Geriatric Psychiatry. 2019;27:213-36.
[Crossref] [Google Scholar] [PubMed]
- Herrera JE, Niehaus WN, Whiteson J, Azola A, Baratta JM, et al. Multidisciplinary collaborative consensus guidance statement on the assessment and treatment of fatigue in Postacute Sequelae of SARS-CoV-2 infection (PASC) patients. Pm & R. 2021;13:1027.
[Crossref] [Google Scholar] [PubMed]
- He J, Yang L, Pang J, Dai L, Zhu J, et al. Efficacy of simplified-cognitive behavioral therapy for insomnia (S-CBTI) among female COVID-19 patients with insomnia symptom in Wuhan mobile cabin hospital. Sleep and Breathing. 2021;25:2213-9.
[Crossref] [Google Scholar] [PubMed]
- Li J, Li X, Jiang J, Xu X, Wu J, et al. The effect of cognitive behavioral therapy on depression, anxiety, and stress in patients with COVID-19: A randomized controlled trial. Frontiers in psychiatry. 2020;11:580827.
[Crossref] [Google Scholar] [PubMed]
- Dana PM, Sadoughi F, Hallajzadeh J, Asemi Z, Mansournia MA, et al. An insight into the sex differences in COVID-19 patients: What are the possible causes?. Prehospital and disaster medicine. 2020;35:438-41.
[Crossref] [Google Scholar] [PubMed]
- Mazza MG, Palladini M, De Lorenzo R, Magnaghi C, Poletti S, et al. Persistent psychopathology and neurocognitive impairment in COVID-19 survivors: Effect of inflammatory biomarkers at three-month follow-up. Brain, behavior, and immunity. 2021;94:138-47.
[Crossref] [Google Scholar] [PubMed]
- Amalakanti S, Arepalli KV, Jillella JP. Cognitive assessment in asymptomatic COVID-19 subjects. Virusdisease. 2021;32:146-9.
[Crossref] [Google Scholar] [PubMed]
- Hampshire A, Trender W, Chamberlain SR, Jolly AE, Grant JE, et al. Cognitive deficits in people who have recovered from COVID-19. EClinicalMedicine. 2021;39.
[Crossref] [Google Scholar] [PubMed]
- Henneghan AM, Lewis KA, Gill E, Kesler SR. Cognitive impairment in non-critical, mild-to-moderate COVID-19 survivors. Frontiers in Psychology. 2022;13:770459.
[Crossref] [Google Scholar] [PubMed]
- Viveiros A, Rasmuson J, Vu J, Mulvagh SL, Yip CY, et al. Sex differences in COVID-19: Candidate pathways, genetics of ACE2, and sex hormones. American Journal of Physiology-Heart and Circulatory Physiology. 2021;320:H296-304.
[Crossref] [Google Scholar] [PubMed]
- Swärd P, Edsfeldt A, Reepalu A, Jehpsson L, Rosengren BE, Karlsson MK. Age and sex differences in soluble ACE2 may give insights for COVID-19. Critical care. 2020;24:1-3.
[Crossref] [Google Scholar] [PubMed]
- Kesler SR, Henneghan AM, Prinsloo S, Palesh O, Wintermark M. Neuroimaging based biotypes for precision diagnosis and prognosis in cancer-related cognitive impairment. Frontiers in Medicine. 2023;10:1199605.
[Crossref] [Google Scholar] [PubMed]
- Mizrahi B, Sudry T, Flaks-Manov N, Yehezkelli Y, Kalkstein N, et al. Long COVID outcomes at one year after mild SARS-CoV-2 infection: Nationwide cohort study. bmj. 2023;380.
[Crossref] [Google Scholar] [PubMed]
- Ballouz T, Menges D, Anagnostopoulos A, Domenghino A, Aschmann HE, et al. Recovery and symptom trajectories up to two years after SARS-CoV-2 infection: Population based, longitudinal cohort study. Bmj. 2023;381.
[Crossref] [Google Scholar] [PubMed]
- Hartung TJ, Bahmer T, Chaplinskaya-Sobol I, Deckert J, Endres M, et al. Predictors of non-recovery from fatigue and cognitive deficits after COVID-19: A prospective, longitudinal, population-based study. EClinicalMedicine. 2024;69.
[Crossref] [Google Scholar] [PubMed]




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.