An Open Label Clinical Study to Evaluate the Safety and Gastrointestinal Tolerance of ONS in Hospitalized Patients Requiring Enteral Tube Feeding
Received: 22-Jul-2021 Accepted Date: Aug 05, 2021 ; Published: 30-Jul-2021
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Background: The present study aimed to investigate the compliance of the Oral Nutritional Supplement (ONS) in enteral feeding protocol on the basis of GI Tolerance and improvement in patient outcomes, including safety parameters. Design: It is a 1 month, prospective, open label, investigatorinitiated study conducted between 6 Jan 2020 to 31 January 2021. Methodology: Participants of either sex, aged ≥ 18 years, hospitalized for minimum two days, requiring enteral feeding were given two feeds of ONS (45 gm diluted in 100 mL of water) along with four standard kitchen feeds at different timings. Primary endpoint was to monitor continuous GI and stomach tolerance and effect o n G astric R esidual Volume (GRV). Secondary endpoints were change in BMI, Serum albumin levels, and adverse events monitoring. Data was analyzed descriptively using paired ttest and p value of <0.05 was considered statistically significant. Results: Fifteen (12 males, 3 females, mean age: 46.3 ± 8.9 years) participants were enrolled. None of participants reported gastric intolerance. Continuous GI tolerance presented patient compliance for the ONS with Gastric Residual Volume (GRV) within the limits (<500 ml) for all participants. Significant gradual improvement in BMI (p<0.0001) and serum albumin levels (p=0.0001) were reported in hospital when fed with ONS supplement administered with hospital kitchen feed. Conclusion: Results of current study suggest that ONS is safe and tolerable product for patients requiring enteral feeding, however larger clinical studies (with statistically significant sample size) can be planned to see broader aspects of enteral feeding.
Keywords
Oral nutritional supplement; Gastrointestinal tolerance; Serum bilirubin; BMI after ONS; Enteral tube feeding
Introduction
In modern critical care, the paradigm of ‘therapeutic nutrition’ is replacing traditional ‘supportive nutrition’. Standard enteral formulas meet basic macro- and micronutrient needs; therapeutic enteral formulas meet these basic needs and also contain specific pharmaco nutrients that may attenuate hyper inflammatory responses, enhance the immune responses to infection, or improve gastrointestinal tolerance. Choosing the right enteral feeding formula may positively affect a patient’s disease outcome; targeted use of therapeutic formulas can reduce the incidence of infectious complications and shorten the duration of hospital stays.
Under nutrition in hospital patients is associated with impaired immune responses, impaired wound healing and reduced muscle strength. As a result, these undernourished patients cope less well with modern medical and surgical interventions and length of stay in hospital increases on an average by five days, incurring approximately 50% greater costs. Hospitals should therefore aim to provide sufficient amounts of adequate nutrition to all patients. In the majority of all patients this can be provided through regular catering services, good care and physical help with eating, whenever necessary. However, many ICU hospital patients do not, or cannot, eat adequately and some of them benefit from oral supplements or even active nutritional support provided through Enteral Tube Feeding (ETF) temporarily or for a longer period of time.
Enteral feeds are nowadays formulated with active nutrients aiming to reduce oxidative stress and damage to cells and tissues, modulate inflammation, enhance beneficial responses to stress and improve feeding tolerance. The present study aimed to investigate whether enteral feeding protocol was to assess Oral Nutritional Supplement (ONS) compliance on the basis of GI Tolerance and if enteral feeding was able to improve patient important outcomes and was safe for use on the basis of adverse events reported.
Method
The current study was a 1 month, prospective, open label, investigator-initiated study conducted from January 06, 2020 to January 31, 2020. Fifteen adult participants, of either sex, aged 18 years and above, hospitalized patients requiring and able to tolerate enteral tube feeding, with minimum 2 days of hospital stay, were included in the study. Participants <18 years of age, receiving tube feeding prior to hospitalization, having allergy to ONS, presenting any evidence of organ dysfunction or any clinically significant deviation from the normal, in physical or clinical determinations or having a history of renal, hepatic, cardiovascular, respiratory, skin, hematological, endocrine, neurological or gastrointestinal diseases, were excluded. Data of participants receiving 2 Feeds of ONS (MaxvidaTM HPHF) (45 gm diluted in 100 mL of water) was administered per day at 0800 hrs and 1600 hrs. The hospital feed included 350 ml each of a standard kitchen feed administered at 1000 hrs, 1200 hrs, 1800 hrs and 2000 hrs (1 ml of the hospital kitchen feed translated into 1 kcal). The Gastric Residual Volume (GRV) was measured at 0730 hrs, 0900 hrs, 1530 hrs, 1700 hrs.
The study was performed in compliance with the principles of the Declaration of Helsinki, in accordance with the international conference of harmonization guideline for good clinical practice, and in accordance with applicable regulatory requirements. All participants provided a written informed consent.
Endpoints
The primary endpoint was to monitor continuous GI tolerance and the following parameters were included: the number of diarrhea free days, stomach irritation, regurgitation, abdominal bloating, vomiting and GRV (>500 ml). Secondary endpoints measured were change in BMI during pre and post intervention of the study product, change in serum albumin during pre and post intervention of the study product, adverse event and serious adverse events were monitored. Adverse event monitoring included vital signs and potential abnormalities in the laboratory parameters.
Statistical Analysis
Descriptive statistics for continuous variable and frequency with percentage for categorical variables were performed. Data were analyzed and the mean data along with the Standard Deviation (SD) were subjected to statistical analysis using paired t-test. A ‘p’ value<0.05 has been marked statistically significant difference from the pre values.
Results
Total 15 participants having normal physical examination were enrolled in the study; of these all participants completed the study [Figure 1]. As a standard of care, hospital maintains a minimum calorie requirement of 1600 kcal/day for hospitalized patients. Hospital kitchen feed: The calorie content of Hospital kitchen feed was 1 kcal/ml. Four servings of 350 ml each of hospital feed translated to 1400 kcal of energy provided to participants per day.
ONS nutritional profile: Nutritional profile obtained below shows daily energy requirements were met by 70 g (45g*2) of ONS (336 kcal/day) [Table 1].
| Nutrients | Unit | Per 100 g | Per 45 g | % RDA |
|---|---|---|---|---|
| Energy* | Kcal | 374 | 168 | 15 |
| Fat* | g | 2 | 0.9 | 7 |
| Saturated fat | g | 0.2 | 0.09 | ? |
| MUFA | g | 0.4 | 0.18 | ? |
| PUFA | g | 1.4 | 0.63 | ? |
| Omega-6 | g | 0.8 | 0.35 | ? |
| Omega-3 | g | 0.4 | 0.18 | ? |
| DHA | mg | 253 | 113.8 | ? |
| EPA | mg | 128 | 57.6 | ? |
| Trans fatty acid | g | 0 | 0 | ? |
| Cholesterol | mg | 0.7 | 0.31 | ? |
| Carbohydrates | g | 61 | 27 | ? |
| Sugar (Sucrose) | g | 5 | 2.25 | ? |
| Dietary fiber## | g | 11.1 | 5 | 33 |
| Soluble fiber | g | 10 | 4.5 | ? |
| Insoluble fiber | g | 1.1 | 0.5 | ? |
| Prebiotic | g | 6.7 | 3 | ? |
| Protein* | g | 28 | 12.6 | 42 |
| L-Valine | g | 1.5 | 0.66 | |
| L-Isoleucine | g | 1.4 | 0.64 | |
| L-Leucine | g | 2.4 | 1.07 | |
| L-Glutamine & L-Glutamic acid | g | 5.6 | 2.5 | ? |
| L-Arginine | g | 2.2 | 1 | ? |
| Vitamins^^ | ||||
| Vitamin A* | mcg | 200 | 90 | 30 |
| Vitamin D* | IU | 133.3 | 60 | 30 |
| Vitamin E* | IU | 11.1 | 5 | 100 |
| Vitamin K* | mcg | 18.3 | 8.25 | 30 |
| Vitamin C* | mg | 44.4 | 20 | 100 |
| Folic acid*^ | mcg | 37 | 16.65 | 28 |
| Vitamin B1 (Thiamin)* | mg | 0.4 | 0.18 | 30 |
| Vitamin B2 (Riboflavin) * | mg | 0.5 | 0.21 | 30 |
| Vitamin B3 (Niacin)* | mg | 5.3 | 2.4 | 30 |
| Vitamin B6* | mg | 0.7 | 0.3 | 30 |
| Vitamin B12* | mcg | 0.42 | 0.19 | 37 |
| Pantothenic acid** | mg | 1.7 | 0.75 | 30 |
| Biotin** | mcg | 10 | 4.5 | 30 |
| Choline | mg | 150 | 67.5 | ? |
| Minerals$ | ||||
| Iron* | mg | 7 | 3.15 | 37 |
| Calcium* | mg | 200 | 90 | 30 |
| Phosphorus * | mg | 200 | 90 | 30 |
| Magnesium * | mg | 113.3 | 51 | 30 |
| Zinc * | mg | 4 | 1.8 | 30 |
| Iodine * | mcg | 50 | 22.5 | 30 |
| Copper* (AI) | mcg | 566.7 | 255 | 30 |
| Selenium* | mcg | 44.4 | 20 | 100 |
| Chromium* (AI) | mcg | 16.7 | 7.5 | 30 |
| Manganese* (AI) | mg | 1.3 | 0.6 | 30 |
| Molybdenum** | mcg | 15 | 6.75 | 30 |
| Sodium* | mg | 460 | 207 | 20 |
| Potassium* | mg | 360 | 162 | 9 |
| Chloride## (AI) | mg | 100 | 45 | 5 |
*: ICMR RDA 2010, **: Codex (CAC/GL 2-1985- Guidelines on nutrition labelling), ## : Adequate Intake given by Food and Nutrition board, IOM, ?: RDA not established in ICMR/WHO, ^: 1 mcg Folic Acid=1.7 mcg Dietary Folate Equivalent, AI: Adequate Intake
Table 1: Nutritionalprofile for ONS.
Demographics
Mean age and BMI of the study participants were 40.13 ± 12.5 years and 27.6 ± 16.0 kg/m2, respectively. Study population included more males (n=12; 80.0%) than females (n=3; 20.0%) as shown in Table 2. 11 participants (73.33%) were <50 years of age.
| Age and gender | No.(n=15) | Percentageof Participants (%) | ||
|---|---|---|---|---|
| Age in years | ||||
| <50 | 11 | 73.33 | ||
| = 50 | 4 | 26.66 | ||
| Mean age ± SD (Range) | 40.13 ± 12 (23.0-66.0) | |||
| Gender | ||||
| Male | 12 | 80 | ||
| Female | 3 | 20 | ||
Table 2: Demographic distribution of study participants.
Primary endpoints
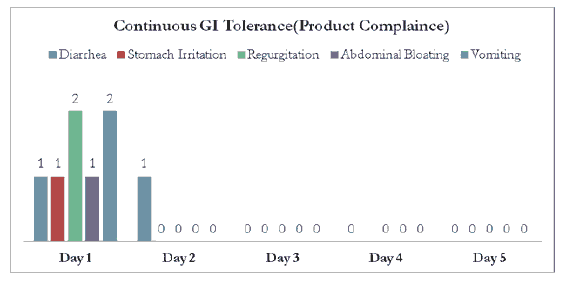
Continuous GI tolerance presented efficacious patient compliance for the ONS [Figure 2]. Total of 2 participants (13.33%) reported diarrhoea. Among them, 1 participant (6.66%) reported diarrhoea on Day 1 and the other (6.66%) on day 2. Rests of the days were diarrhoea free days for all the participants. 2 participants (13.33%) reported regurgitation on day 1 and other 2 participants reported vomiting on day 1. Only 1 participant (6.66%) reported stomach irritation on day 1 and 1 participant (6.66%) reported abdominal bloating on day 1. All other participants tolerated study product without any adverse effect throughout the study period.
GRVs were within the limits (<500 ml) for continual tube feeding in all participants showing positive signs of GI tolerance for feed [Figure 3]. Distribution of GRV was 341.50,345.17, 347.80, 357.79 and 340.77 ml on Day 1, 2, 3, 4 and 5 respectively.
Secondary endpoints
Following are the results for comparison of pre and post mean values of BMI [Table 3] and mean change in serum albumin level from Day 1 to subsequent days [Table 4] observed during the clinical study period. Mean value for BMI was 18.43 (n=15) at day 1 which increased to 18.69 (n=15) at the end of hospitalization with 1.41% increase and statistical significant p value (p value<0.0001, comparison done using paired t test). Mean value for serum albumin levels was 3.66 g/dl (n=15) at day 1 which increased to 4.26 g/dl at the end of hospitalization with 16.39% increase with statistical significant p value (p value<0.0001, comparison done using paired t test).
| Day 1 | End of Hospitalization/ Day 5 (Whichever is earlier) | |||||
|---|---|---|---|---|---|---|
| Height | Weight | BMI | Height | Weight | BMI | |
| Mean | 165.9 | 50 | 18 | ## | # | # |
| Median | 162.5 | 50 | 19 | ## | # | # |
| SD | 14.42 | 6.8 | 1.9 | 14 | 7 | 2 |
| Min | 152 | 45 | 12.2 | 152 | 45 | # |
| Max | 192 | 65 | 19.5 | 192 | 66 | # |
| Mean (SD) change: Day 1 to end of hospitalization/day 5 (whichever is earlier) | 0.26 (0.01) | |||||
| P value based on paired t test | <0.0001* | |||||
| Percent Change: Day 1 to end of hospitalization/ day 5 (whichever is earlier) | 1.41% | |||||
Table 3: Change in BMI during pre and post intervention of ONS.
| Day 1 | End of hospitalization/ Day 5 (Whichever is earlier) | |
|---|---|---|
| Mean | 3.66 | 4.26 |
| SD | 0.39 | 0.36 |
| Median | 3.6 | 4.2 |
| Min | 3.1 | 3.8 |
| Max | 4.4 | 4.9 |
| Mean change (SD) | 0.6 (-0.03) | |
| P value based on paired t test | 0.0001099* | |
| % Change | 16.39 |
Table 4: Change in serum albumin from Day 1 to the end of hospitalization.
Increase in BMI and serum albumin levels were reported to improve gradually while stay of participants in hospital when fed with ONS supplement administered with hospital kitchen feed. No hematological adverse events were reported by any of the participants during the study.
Safety
No severe Adverse Event (AE) was reported. Of the AEs observed, two participants experienced diarrhea (2; 13.33%) during the study, which was reported as unrelated to the study product. Other AEs reported during this study were regurgitation (2; 13.33%), stomach irritation (1; 6.66%), vomiting (1; 6.66%) and abdominal bloating (1; 6.66%).
Discussion
Nutrition therapy is of paramount importance for critically ill patients, because critical illness is usually associated with catabolic state when energy requirements are increased. The term “nutrition support” has been changed to “nutrition therapy”, indicating increased awareness of the importance of nutrition for the critically ill in the medical community. Nutrition can be delivered enterally or intravenously. There is large body of evidence favoring Enteral Nutrition (EN) to Parenteral Nutrition (PN) owing to EN benefits such as comparative convenience, better safety and efficacy. [1,2] PN is associated with nosocomial infection and prolonged intensive care length of stay, but not mortality. [3,4] The most-updated nutrition support guideline recommends that EN should be started within 24 to 48 hours after admission to minimize medical complications, while PN can be withheld for seven days depending on the risk of malnutrition. [5]
Despite the importance of early initiation of EN, it is reported that energy requirements of critically ill patients are far from being reached, [6] mainly due to delayed initiation of EN. [7] Underfeeding is associated with detrimental clinical outcomes including prolonged length of stay, infection, financial cost, impaired wound healing, and increased morbidity and mortality. [6,8] Factors associated with inadequate enteral feeding include delayed initiation of EN, slow advancement of infusion rate, gastrointestinal dysfunction, under prescription, incomplete delivery of prescribed nutrition, and frequent interruption of EN. [6,9]
The present study was conducted to evaluate the safety and compliance of ONS in hospitalized patients requiring enteral tube feeding and if enteral feeding was able to improve patient important-outcomes and was safe for use. Common gastrointestinal symptoms seen in patients receiving EN are nausea, abdominal bloating and delayed gastric emptying. [10] Assessment of GI Tolerance of ONS in our study reported diarrhea in 13.33 cases, stomach irritation inn 6.66%, regurgitation in 13.33%, abdominal bloating in 6.66% and vomiting in 6.66% of cases. Rest of the patients tolerated the ONS without any adverse effects throughout the study period. Mean GRV volume on all days for all patients was <500 which allowed to continue the product enteral feeding throughout the study. Formulation associated intolerance can occur due to any of the constituents or owing to prolonged usage of the ONS. This stresses the importance of ingredient consistency, mode and frequency of ONS administration as well as time, volume, type, rate and delivery site of feed. [10,11] ONS compliance (GI tolerance) results are in line with previous published literatures for enteral feeding [12,13] and can be considered as safe and tolerable product of patients requiring enteral feed.
Concurrently other parameters like change in BMI, change in serum albumin during pre and post intervention of the ONS and adverse event monitoring was also done. Mean value for BMI increased by 1.41% and mean value for serum albumin levels increased by 16.39% at the end of hospitalization with statistical significant p-value (p value<0.0001) suggesting improvement in nutritional status of patients. No hematological adverse events were reported by any of the subject during the study. All other adverse events (n=8) reported were part of evaluation of primary objective of the study and were related to GI tolerance. For critically ill patients, it is essential to opt for a high-protein formula containing approximately 2 grams of protein per kilogram of bodyweight which is administered daily. [2] A high protein enteral formula constituting more than 15% of total calorie content is indicated in catabolic conditions and wound healing whereas a fibrous feed containing 5-15 gm of fiber per liter is indicated for adjustment of intestinal functions in hospitalized patients. [14] The high-protein and high-fiber feature of the ONS led to significant improvement in BMI and serum albumin levels which might have further contributed to the ONS tolerance and safety. In a similar study by, the authors observed that hydrolysed proteins and more easily digestible fats including medium-chain triglycerides tend to supply protein and fats to the best suitability of patients with impaired gastrointestinal function. [15] A formula with fiber would be contraindicated in hemodynamically unstable patients and the ones who are at risk for bowel ischemia. [16]
Some of these factors can be improved with enteral feeding protocols, therefore preventing underfeeding of critically ill patients. There was evidence that implementation of enteral feeding protocol was associated with more EN intake alone, and early initiation of EN. [17-19] However, there is no evidence suggesting the reduction of mortality or other patient-important outcomes.
Conclusion
Results of current study suggest that ONS MaxvidaTM HPHF is safe and tolerable feed for patients requiring enteral feeding, however larger clinical studies (with statistically significant sample size) can be planned to see broader aspects of enteral feeding.
Ethics Compliance
All ethical approvals required for the study was obtained before the start of the trial.
Conflict of Interest
None
Funding
The work was supported by Signutra®.
Acknowledgements
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work and have given final approval for the version to be published. The authors wish to thank participating investigators for contributing participants’ data for this study. The authors also acknowledge Dr. Atul Mishra, Ms. Aparna Tandon and Dr. Punit Srivastava from Medi Ception Science Pvt Ltd (www.mediception. com). for providing writing assistance, which were funded by Signutra®.
REFERENCES
- Heidegger CP, Darmon P, Pichard C. Enteral vs. parenteral nutrition for the critically ill patient: A combined support should be preferred. Curr Opin Crit Care. 2008;14:408-14.
- Nguyen DL. Guidance for supplemental enteral nutrition across patient populations. Am J Manag Care. 2017;23:210-219.
- Elke G, van Zanten AR, Lemieux M, McCall, Jeejeenhoy KN, Kott M, et al. Enteral versus parenteral nutrition in critically ill patients: An updated systematic review and meta-analysis of randomized controlled trials. Crit Care. 2016;20: 117.
- Netto R, Mondini M, Pezzella C, Romani L, Lucignano B, Pansani L, et al. Parenteral nutrition is one of the most significant risk factors for nosocomial infections in a pediatric cardiac intensive care unit. JPEN J Parenter Enteral Nutr. 2015.
- Taylor BE, McClave SA, Martindale RG, Warren MM, Johnson DR, Braunschweig C, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (ASPEN). Crit Care Med. 2016;44: 390-438.
- Kim H, Stotts NA, Froelicher ES, Engler MM, Porter C. Why patients in critical care do not receive adequate enteral nutrition? A review of the literature. J Crit Care. 2012;27:702-13.
- Turner P. Providing optimal nutritional support on the intensive care unit: key challenges and practical solutions. Proc Nutr Soc. 2010;69: 574-81.
- Caccialanza R, Klersy C, Cereda E, Cameletti B, Bonoldi A, Bonardi C, et al. Nutritional parameters associated with prolonged hospital stay among ambulatory adult patients. CMAJ. 2010;182:1843-9.
- Oshima T, Pichard C. Parenteral nutrition: Never say never. Crit Care. 2015;19(Suppl 3):S5.
- Stroud M, Duncan H, Nightingale J; British society of gastroenterology. guidelines for enteral feeding in adult hospital patients. Gut. 2003;52(Suppl 7):vii1-vii12.
- Kakkar A. Mehndiratta C, Agrawal T. Enteral tube feeding: Safety and tolerance of nutritional supplements in hospitalized patients. Int Clin Med. 2019;3; 1-4
- Lottes SM. Nutrition Support Protocols and their influence on the delivery of enteral nutrition: a systematic review. Worldviews Evid Based Nurs. 2014;1:194-9.
- Pinilla JC, Samphire J, Arnold C, Liu L, Thiessen B. Comparison of gastrointestinal tolerance to two enteral feeding protocols in critically ill patients: A prospective, randomized controlled trial. J Parenter Enteral Nutr. 2001;3:166-7
- Hassan GM, Nikooyeh B, Motamed S, Neyestani RT. Efficacy of commercial formulas in comparison with home-made formulas for enteral feeding: A critical review. Med J Islam Repub Iran. 2017;5: 31:55.
- Green B, Sorensen K, Phillips M, Green L, Watson R, McCallum A, et al. Complex enterally tube-fed community patients display stable tolerance, improved compliance and better achieve energy and protein targets with a high-energy, high-protein peptide-based enteral tube feed: Results from a multi-centre pilot study. Nutrients. 2020; 18;12(11):3538.
- Tadlock MD, Hannon M, Davis K, Lancman M, Pamplin J, Shackelford S, et al. Nutritional support using enteral and parenteral methods. Mil Med. 2018; 1;183(suppl-2):153-160.
- Heyland DK, Cahill NE, Dhaliwal R, Sun X, Day AG, McClave SA,et al. C. JPEN J Parenter Enteral Nutr. 2010;34:675-84.
- Doig GS, Simpson F, Finfer S, Delaney A, Davies AR, Mitchell I, et al. Effect of evidence based feeding guidelines on mortality of critically ill adults: a cluster randomized controlled trial. JAMA. 2008;300:2731-41.
- Lottes SM. Nutrition support protocols and their influence on the delivery of enteral nutrition: A systematic review. Worldviews Evid Based Nurs. 2014;11:194-9.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.