Association of Gingival Pigmentation of Different Age Groups Passive Smokers (ETS) with Active Household Smoker in Erbil City/Iraq
2 Conservative Department, Tishk International University, Erbil, Iraq
Received: 24-Dec-2021, Manuscript No. AMHSR-21-45718; Editor assigned: 27-Dec-2021, Pre QC No. AMHSR-21-45718(PQ); Accepted Date: Jan 24, 2022 ; Reviewed: 11-Jan-2022 QC No. AMHSR-21-45718; Revised: 17-Jan-2022, Manuscript No. AMHSR-21-45718(R); Published: 24-Jan-2022, DOI: 10.54608.annalsmedical.2022.22
Citation: Hussein SR, et al. Association of Gingival Pigmentation of Different Age Groups Passive Smokers (ETS) with Active Household Smoker in Erbil City/Iraq. Ann Med Health Sci Res. 2022;12:1-7.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
To determine the relationship of gingival pigmentation of different passive smoker age groups (both genders) with active household smokers in Erbil city/Iraq. Passive smoking is also called ETS (Environmental tobacco smoke) is the result of spontaneous cigarette burning, and contrarily, the result of the side-stream of cigarette smoke, as well as the smoke exhaled by active smokers. ETS produces numerous side effects including oral cavity of people who do not smoke like oral mucosal pigmentation, which is fundamentally, cigarette smoking affecting smile and esthetics. Health personnel should clearly present the data concerning the adverse results of smoking, in addition to the dangers to exposure to environmental tobacco smoke. A total of 400 patients attending different dental clinics in Erbil city, participated in the study, 170 passive smokers case group, and 230 non-smoker control group (age 18-70 years) (112 male, 58 females for case group) and (128 male, 102 females for control group) were included in the study sample. All the participants were assessed for gingival pigmentation and were medically free of any systematic disease. The additive number and percentage of control group with the total of pigmentation-lack (male 101, 25.25%) (female 95, 23.75%) (total 196, 49%) was higher than case group (male 85, 21.25%) (female 49, 12.25%) (total 134, 33.5%) with highly significant difference at P=0.000 level. For gingival pigmentation case group in its specific details 36 (male 28, 7%)(female 8, 2%) was higher in number and percentage than control group 34 (male 27, 6.75%) (female 7, 1.75%) without significant difference at P 0.1322 but for total case group (36, 9%) was higher than total control (34, 8.5%) with statistically significant difference at P 0.0322. Concerning severity of gingival pigmentation grade 0: Total control group number and percentage 196 (49%) was higher than total case number and percentage 133 (33.25%) with highly statistically significant difference between them P=0.0013 level. For Grade 1: There was no statical significant difference between total control and case groups for gingival pigmentation at P 0.5 level. For Grade 2: Total case group number and percentage 15 (3.75%) was higher than total control group 12 (3%) with statistical significant difference at P=0.06 level concerning distribution of gingival pigmentation according to age relating factor higher number and percentage were seen at age (30-39 years) for case group more than control group with highly significant difference at P 0.0012, followed by age (40-49 years) case group was higher in number and percentage more than control group with highly significant difference at P=0.000 level, while for age (18-29 years) control group number and percentage was higher than case group with significant difference at P=0.029 level followed by group 50 years control group number and percentage was higher than case group with highly significant difference at P=0.000 level.
Keywords
Passive smoking; ETS; Secondhand smoker; Gingival pigmentation
Introduction
Smoking is seen to have many adverse effects. The World Health Organization (WHO) classifies smoking as a chronic, progressive disease which is contagious, it’s also considered to be a necrobiotic addiction. [1]Nicotine addiction does not cause changes in behavior or functioning of a smoker, but it negatively influences the health of passive smokers like oral mucosa pigmentation which is clinically manifested as a result of tobacco smoking, [1]which is mainly due to the accumulation of melanin (smoker’s melanosis or oral mucosal pigmentation). [2]
Passive smoking (is so called ETS-Environmental tobacco smoke) which means accompanying smokers negatively influences the health of passive smokers. ETS on the other hand, is the result of spontaneous cigarette burning and fundamentally, the result of the side-stream of cigarette smoke, as well as the smoke exhaled by active smokers.
Pigmentation can occur in different areas of oral cavity such as gingiva, palate, labial mucosa, ventral surface of the tongue and rarely floor of the mouth and attached gingiva is the most involved. [3-5]Smoking [5-9]cigarettes a day appeared to be sufficient to cause gingival melanin pigmentation. [6]Secondhand smoke can compromise the health of individuals at all ages. [7,8]
The aim of this study was to determine the relationship of gingival pigmentation of different passive smokers age groups (both genders with active household smokers to protect nonsmokers in their environment from the adverse effects of exposure to environmental tobacco.
Patients and Methods
A total of 400 subjects participated in the study, who reported to different governmental and private clinics in Erbil city, randomly selected, in the age group of 18-70 years, informed consent was obtained from all subjects and the study was approved by institutional ethical committee (Ethical approval no.2986 on August 4th, 2021). The sample was, 170 subjects’ passive smokers case group, who at least had one member in their families a smoker or smoking a minimum 6-10 cigarettes/day and smoking at least 6 cigarettes or more at home once a day in their presence in the past 6 months, and 230 subject’s nonsmoker control group who didn’t have a smoker in their family.
Distribution of age and gender were included in the study, participants were divided into subgroups by their age (10 years interval), (case group: 112 males, 58 females), (control group: 128 males, 102 males) were included in the study sample at the base line.
All study sample subjects (control and case groups) were completely healthy and didn’t use any medications that induce pigmentation.
Inclusion criteria
1.Patient aged 18-70 years
2.Healthy patients
Exclusion criteria
1.Patients with drug induced pigmentation
2.Patients who were diagnosed with chronic periodontitis
All participants were assessed for gingival pigmentation by using Takashi et al. assessment index in 2005, [9]to measure gingival melanin pigmentation. Mouth mirror and sickle shaped probe were used for examination.
Takashi et al. index grades were as follows: [10]
1.Score 0: No pigmentation
2.Score 1: Solitary unit(s) of pigmentation in papillary gingiva without extension between neighboring solitary units.
3.Score 2: Formation of continuous ribbon extending from neighboring solitary units.
Statistical Analysis
Data were collected and entered in the computer, analyzed by using appropriate data system which is called (SPSS) Statistical Package for Social Science version 22, the results will be compared between participants with different variables with statistical significance level of <0.05 level, they will be presented as percentages in Tables, Figures using chi-square test.
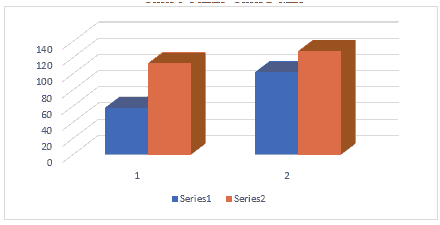
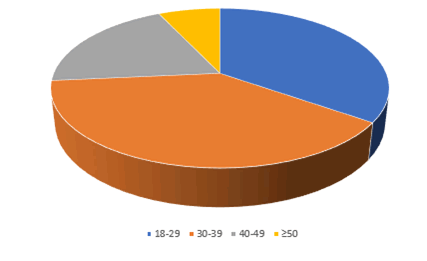
Table 1 revealed that a total sample of 400 participants were divided into 2 groups: case group comprised 170 (42.5%) participants with a passive family smoker, male 112 (28.25%), female 58 (14.5%), while control group number, was 230 (57.5%) participants with a history of no family smoking, male comprised 128 (32%) of the sample and female 102 (25.5%) of the sample as it is shown in Figure 1.
| Variable | Case 170(42.5%) | Control 230(57.5%) | Total 400(100%) | |||||
|---|---|---|---|---|---|---|---|---|
| No. (%) Male 112 (28.25%) | No. (%) Female 58 (14.5%) | No. (%) Male 128 (32%) | No. (%) Female 102 (25.5%) | Males of case and control | Females of case and control | |||
| Gingival pigmentation | Male case | Female case | Male control | Female control | Males and females of case and control | Total case | Total control | ***P-Value |
| Yes (%) | 28 (7%) | 8 (2%) | 27 (6.75%) | 7 (1.75%) | 0.132 | 36 (9%)** | 34 (8.5%)** | ** 0.0322 |
| No (%) | 85 (21.25%) | 49(12.25%) | 101 (25.5%) | 95 (23.75%) | 134 (33.50%) | 196 (49%) | * 0 | |
| * p-value | *0.000 | 0 | * 0.000 | 0 | 0 | |||
| * No. and % of control group without pigmentation, male 101 (25.25%), female 95 (23.75%) and the total 196 (49%) was more than case group, male 85 (21.25%), female 49 (12.25%) & total 134 (33.5%) with highly significant difference at P= 0.000 level. ** Statistically significant difference between total case 36 (9%) more than total control group 34 (8.5%) at P=0.032 * * * Male 28 (7%), female 8 (2%) case group was more than control group, male 27 (6.75%), female 7 (1.75%) with no significant difference at P= 0.1322. |
||||||||
Table 1: Number and percentage distribution of total sample groups of both genders according to presence and absence of gingival pigmentation.
The additive number and % of control group without pigmentation including all its specific details, male 101 (25.25%), female 95 (23.75%) plus total 196 (49%) was higher than case study specific details male 85 (21.25%), female 49 (12.25%), plus total 134 (33.5%) with highly significant difference at P=0.000 level as it is shown in Figure 1.
For gingival pigmentation case group including its specific details, male 28 (7%), female 8 (2%) plus total 36 (9%) was more than control group male 27 (6.75%), female 7 (1.75%) plus total 34 (8.5%) with statistically significant difference at P=0.032 for the total, but with non-significant difference for the details at P=0.1322 level as it is shown in Figure 1.
Table 2 showed that comparing total control group number and percentage 196 (49%) for grade 0 gingival pigmentation was higher than total case group 134 (33.25%) with highly significant difference between them at P=N 0.0013
| Variable | Grades of gingival pigmentation | |||
|---|---|---|---|---|
| NO.170 (%42.5) | Grade 0 | Grade 1 | Grade 2 | |
| Brother | 68 (17%) | 55 (13.75%) | 6 (0.02%) | 7 (1.75%) |
| Father | 57 (14.25%) | 39 (9.75%) | 13 (3.25%) | 5 (1.25%) |
| Husband | 24 (6%) | 22 (5.5%) | 1 (0.25%) | 1 (0.25%) |
| Mother | 14 (3.5%) | 12 (3%) | 1 (0.25%) | 1 (0.25%) |
| Sister | 7 (1.75%) | 5 (1.25%) | 1 (0.25%) | 1 (0.25%) |
| Total case | 170 (42.5%) | 134 (33.25%)* | 22 (5.5%) | 15 (3.75%) *** |
| Control | 230 (57.5%) | 196 (49%)* | 22 (5.5%) | 12 (3%) *** |
| P-value | *0.0013 | **0.5 | ***0.06 | |
| * Number and percentage of total control group 196 (49%) grade 0 gingival pigmentation was more than total case 134 (33.25%) with highly significant difference at P 0.0013. ** No statistically significant difference between both case and control group for grade 1 gingival pigmentation at P 0.5 level. *** Number and percentage of total case group 15 (3.75) % grade 2 gingival pigmentation was more than total control group 12 (3%) with statistically significant difference between them at 0.06 level. |
||||
Table 2: Distribution number and percentage of total both groups in relation to grades of passive gingival pigmentation.
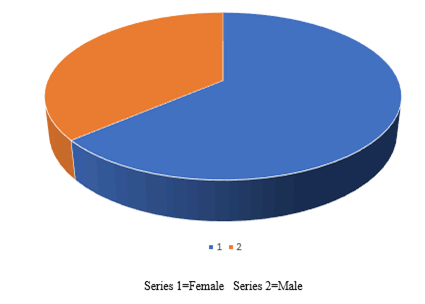
While comparing total case group number and percentage 15 (3.75%) for grade 2 gingival pigmentation was higher than total control 12 (3%) with statistically significant difference between them at P=0.06 level. There was no statistically significant difference between both groups for grade 1 gingival pigmentation at P=0.5 level as it is shown in Figure 2.
The order of family members according to the distribution number and percentage of both groups in relation to grades of passive gingival pigmentation was for brother, father, husband, mother and sister respectively. Except for grade 1, father order occupied the highest percentage followed by brother order.
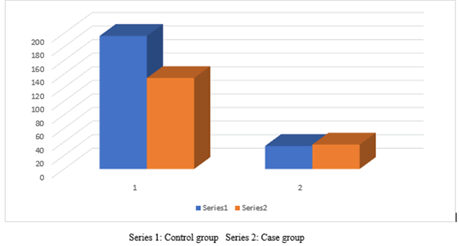
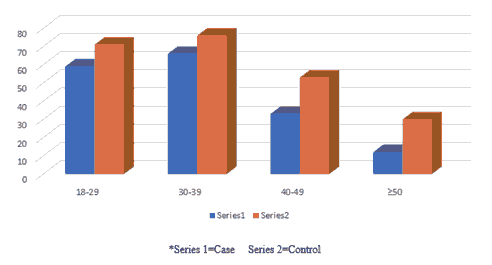
Table 3 revealed the number and percentage of case and control group according to their different age groups, the higher number and percentage of case and control group were seen among 30-39 years, 66 (16.5%), 76 (19%) respectively, and the total is 142 (35.5%) followed by the age 18-29 years, case number 59 (14.75%) and control 71 (17.75%) and the total is 142 (35.5%) respectively as it is shown in Figures 3.
| Age groups | Case | Control | Total |
|---|---|---|---|
| No% | No% | No% | |
| 18-29 | 59 (14.75%) | 71 (17.75%) | 130 (32.5%) |
| 30-39 | 66 (16.5%) | 76 (19%) | 142 (35.5%) |
| 40-49 | 33 (8.25%) | 53 (13.25%) | 86 (21.5%) |
| ≥ 50 | 12 (3%) | 30 (7.5%) | 42 (10.5%) |
| Total | NO.170 (42.5%) | 230 (57.5%) | 400 (100%) |
Table 3: Number and percentage distribution of passive and control group according to their age group.
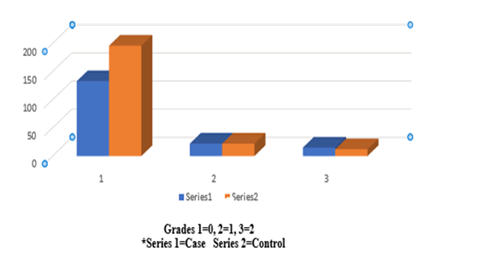
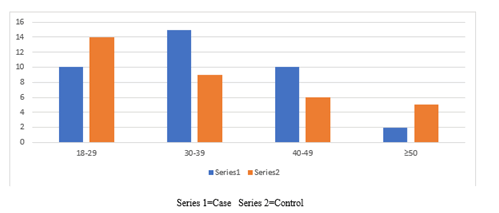
Table 4 showed that the higher number and percentage of passive gingival pigmentation were seen among (30-39) years old for case was 15 (3.75%) more than control 9 (2.25%) with highly significant difference at P=0.0012 level, followed by age 40-49 years old for case group was 10 (2.5%) more than control group 6 (0.02%) with highly statistical significant difference at P=0.000 level while higher number and percentage of gingival pigmentation for control group were seen at age 18-29 years old for control group 14 (3.5%) was more than case group 10 (2.5%) with statistical significant difference at P=0.029 level, followed by age ≥ 50 years old, control group was 5 (1.25%) more than case group 2 (0.5%) with highly significant difference at P 0.000 level as it is shown in Figure 4.
| Age group | Case | Control | P-Value |
|---|---|---|---|
| 18-29 | 10 (2.5%) | 14 (3.5%) | * 0.029 |
| 30-39 | 15 (3.75%) | 9 (2.25%) | ** 0.0012 |
| 40-49 | 10 (2.5%) | 6 (0.02%) | ***0.000 |
| ≥ 50 | 2 (0.5%) | 5 (1.25%) | **** 0.000 |
| Total | 37 (9.25%) | 34 (7.02%) | |
| *Significant difference between case and control group for age (18-29) years 10(25%) and 14(3.5%) at P0.029 level. **Highly significant difference between case and control group for age (30-39) years, 15(3.75%) and 9(2.5%) at P0.0012 level. ***Highly significant difference between case and control group for age (40-49) years, 10(2.5%) and 6(0.02%) at P0.000 level. ****Highly significant difference between case and control group for age ≥50 years, 2(0.5%) and 5(1.25%) at P0.000 level. |
|||
Table 4: Passive gingival pigmentation distribution according to age relating factor among passive and control group.
Discussion
Secondhand smoke can compromise the health of individual at all ages. [7]Smoker’s melanosis (oral mucosal pigmentation) is among the most common lesions in smoker’s mouth. [11]Smokers’ melanosis occurs in up to 21.5% of smokers [12]It has been reported that melanin pigmentation occurs in all human races and both genders. [13]The degree of pigmentation is directly related to the mechanical, chemical, and physiologic stimulators of melanocytes. [14]It has been reported that gingiva is the most commonly pigmented sites in the oral cavity, pigmentation caused as physiologic pigmentation results in an esthetic concern for the adults leading to formation of black gingiva. [15]
There is an increased production of melanin, which may provide a biologic defense against the noxious agents present in tobacco smoke. [12]There are two original ways for the stimulant materials in cigarette smoke to reach the gingival melanocytes. The first way is from the mucosa saliva and the second way is the systemic route which occurs through blood circulation by breathing through nose affecting the melanocytes indirectly, so the second way is a more acceptable explanation. [3,6,9]
Information on the smoking amount was limited, we were unable to determine the smoking index because subjects were unable to detect the number of cigarettes per day. However, there have been studies that reported gingival pigmentation has no relationship with the number of cigarettes smoked per day, [16,17]our outcomes won’t be denied by these limitations.
Table 1 revealed that total case group with gingival pigmentation 36 (9%) were higher than total control 34 (8.5%) with statistically significant difference at P=0.032, this Figures 5-7 is in accordance with a Turkish study population (Eurasia) which was to be 37% (18) and with Caucasian population study where gingival pigmentation prevalence reported to be 30%. [18,19]
Our result was lower than the Indian population (96%), and Iranian population study (54%), [20]and (15%) among European population. This explains its increased production by melanocytes. [2,5]Epidemiologic studies have demonstrated that secondhand smoke contains higher concentrations of toxic compounds particularly nicotine compared to the same amount of smoked tobacco. [21]
Concerning gender, our study revealed that higher number and percentage of gingival pigmentation was greater in male case group 112 (28.25%) and control group 128 (32%), than female case group 58 (14.5%) and control group 102 (25.5%), this result was in accordance with Balaji study in Madurai/India that found the distribution of gingival pigmentation was more in male than female, [22,23]also it’s in accordance with Oberg et al. study that world-wide 33% of male nonsmokers and 35% of female non smokers were exposed to secondhand smoke in 2004. [23]
Our result contrasted with a study conducted in Iran that found the gingival pigmentation passive smoker prevalence was 27 (54%) and control 14 (28%) greater in female than male. [20]
Table 2 revealed that number and percentage of gingival pigmentation grades among different family members arranged from highest to lowest as the following: brother, father, husband, mother and sister respectively this result is in contrast with the Iranian study [20]that found smoking by the husband at home caused gingival pigmentation in passive smokers. Among family members, a husband and wife usually spend the most time with each other, thus, the effects of exposure to secondhand smoke would be greater on women’s health compared to exposure to the secondhand smoke of parents during childhood. [24]It’s worth noting that smoking by the father is less harmful than that by the mother. [25]
According to our best knowledge, no similar study has investigated the effect of passive smoking on family members’ order.
Concerning grades, our study revealed that the highest percentage of both groups in relation to grades of gingival pigmentation was grade [1]22 (5.5%) for both case and control group, with the order of the brother occupying the highest percentage unlike other grades’ order of family members, with non-significant difference between them, in solitary units, in papillary gingiva without extension between neighbouring solitary units according to Takashi et al. [10]this result is in accordance with Hanioka et al. [9]who reported that gingival pigmentation in passive smokers was in the form of separate, single units. Further studies are required to assess patterns of pigmentation in passive smokers in details.
Table 3 clarified the number and percentage of both groups according to their different age groups. Highest number and percentage of case and control groups were seen among age 30-39 years, 66 (16.5%), 76 (19%) respectively.
Table 4 In relation to age factor, our study revealed that higher number and percentage of passive gingival pigmentation were seen among case group 30-39 years 15 (3.75%) more than control group 9 (2.25%) with highly significant difference at P=0.0012, followed by age 40-49 years case group 10 (2.5%) more than control group 6 (0.02%) with highly significant difference at P=0.000 level, regarding why these effects of smoking on gingival pigmentation were so much marked in young people than others, this may be due to the accumulation of melanin by melanocytes. [26]The activation of melanocytes can trigger the accumulation of melanin. Many studies have reported that young people have high melanocyte activity, so our result is in accordance with Gilhar et al. [27]study and with Tomotak et al. [28]study found that gingival pigmentation induced by smoking was more remarkable in young people than in middle aged people. [28]
This result is in accordance with Chen study 2015 that reported the rate of younger smokers has been on the rise for the past several years in some countries. [29]This shows that oral pigmentation is possible in non-smokers who are near other smokers as well. [17,22,30]
It was also found that Environmental Tobacco Smoke (ETS) impairs the final differentiation of keratinocytes [31]and contributes to the increased gingival pigmentation evaluated based on gingival pigmentation index.
Therefore, anti-smoking education is a very important and pressing issue. In the dental field, describing the effects of smoking on gingival pigmentation is effective in educating patients on smoking cessation. Our findings may therefore expand anti-smoking education to include pointing the effects of smoking on gingival pigmentation. [8,14]
Conclusion
Gingival melanosis is not a pathological problem for most patients, it’s a cosmetic problem, it appears as pigmented lesion in every age and group. The present findings suggested an age-related difference in the smoking induced gingival pigmentation in passive smokers was more significant in younger patients than in older ones, more in male than female, grade 1 was more prevalent in both groups, but the pattern of severity was more in grade 2, gingival pigmentation was more in male than female. Therefore, anti-smoking programs should strongly target younger generations.
References
- World Health Organization. The world health report: Reductioning risk, promoting healthy life. Geneva, World Health Organization. 2002. [Crossref], [Google Scholar], [Indexed]
- Eid HA, Syed S, Soliman AN. The role of gingival melanin pigmentation in inflammation of gingiva, based on genetic analysis. J Int oral Health. 2013;5:1-7. [Crossref], [Google Scholar], [Indexed]
- Cicek Y, Ertas U. The normal and pathological pigmentation of oral mucous membrane: A review. J Contemp Dent Pract. 2003;4. [Crossref], [Google Scholar], [Indexed]
- Soliman MM, Al Thomali, Al shammrani A, El Gazaerly H. The use of soft tissue diode laser in the treatment of oral hyperpigmentation. Int J Health Sci (Qasim). 2014;8:133-40. [Crossref], [Google Scholar], [Indexed]
- Hedin CA, Axell T. Oral melanin pigmentation in 467 Thai and Malaysian people with special emphasis on smoker's melanosis. J Oral Pathol Med. 1991;20:8-12. [Crossref], [Google Scholar], [Indexed]
- Kamile M, Kaharaman GU, Hulya, Cakmak J, Serhat D, Erol S, et al. Smoking status and smoking-related gingival melanin pigmentation in army recruitments. Military Medicine. 2007;172:110. [Crossref], [Google Scholar]
- Vainio H. Is passive smoking increasing cancer risk?. Scand J work Environ Health. 1987;13:193-196 . [Crossref], [Google Scholar], [Indexed]
- Hanioka T, Tanaka K, Ojima M, Yuki K. Association of melanin pigmentation in the gingiva of children with parents who smoke. 2005;116:186-90. [Crossref], [Google Scholar], [Indexed]
- Hedin CA. Smoker's melanosis occurrence and localization in the attached gingiva 1977. Arch Dermatol. 1977;113:1533-1538. [Crossref], [Google Scholar], [Indexed]
- Papally S, Fiala Z, Smejkalova J, Jacob V, Somanthan R. Smoking related systematic and oral diseases. Acta Medica (Hradec Kralove). 2007;50:161-166. [Crossref], [Google Scholar], [Indexed]
- Tadakamadla J, Kumar S, Nagori A, Tibdewal H, Duraiswamy P, Kulkarnis S. Effect of smoking on oral pigmentation and its relationship with periodontal status. Dent Res Journal. 2012;9:112. [Crossref], [Google Scholar]
- Kan JY, Morimoto T, Rungcharassaeng K, Roe P, Smith DH. Gingival biotype assessment in esthetic zone: Visual versus direct measurement. Int J Periodontics Restorative Dent. 2010;30:237-43. [Crossref], [Google Scholar], [Indexed]
- Firoozi MD, Mohammadi RN, Rafieyan S. Effect of environmental tobacco smoke on oral pigmentation: A systematic review. J Oral Health Oral Epidemiol. 2020;9. [Crossref], [Google Scholar]
- Kauzman A, Pavone M, Blanas N, Bradely G. Pigmented lesions of the oral cavity: Review, differential diagnosis, and case presentations. J Cand Dent Assoc. 2004;70:682-3. [Crossref], [Google Scholar], [Indexed]
- Marakoglu K, Gursoy UK, Toker HC, Demirer S, Sezer RE, Marakoglu I. Smoking status and smoke related gingival pigmentation in army recruitments. Mil Med. 2007;172:110-113. [Crossref], [Google Scholar], [Indexed]
- Madani SM, Thomas B. Evaluation of gingival pigmentation in children exposed to and not exposed to environmental tobacco smoking. SRMJ Res Dent Sci. 2014;5:21-25. [Crossref], [Google Scholar]
- Unsal E, Paksoy C, Soykan E, Elhan E, Elhan AH, Sahin M. Oral melanin pigmentation related to smoking in a Turkish population. Community. Dent Oral Epidemiol. 2001;29:272-277. [Crossref], [Google Scholar], [Indexed]
- Babee N, Nooribayat S. Frequency of oral pigmentation in patients referred to Babol dental school. J Qazvin Univ Med Sci. 2011;15:8790. [Crossref], [Google Scholar]
- Elahe MS, Elham MS, Farnaz HF. Relationship of gingival pigmentation with passive smoking in women. 2015;14:107-114. [Crossref], [Google Scholar], [Indexed]
- El-Batran MM, Soliman NL, Mikael FF. Passive smoking and alveolar bone density. Aust J Basi Appl Sci. 2009;3:713-9. [Crossref], [Google Scholar]
- Oberg M, Jaakkola MS, Woodward A, Peruga A, Pruss UA. Worldwide burden of disease from exposure to secondhand smoke: A retrospective analysis of data from 192 countries. 2011; 377:139-146. [Crossref], [Google Scholar], [Indexed]
- Hanioka T, Nakamura E, Ojima M, Tanaka K, Aoyama H. Dental caries in 3-years-old children and smoking status of parents. Pediatric Perinat Epidemial. 2008;22:546-550. [Crossref], [Google Scholar], [Indexed]
- Tanaka K, Miyake Y, Sasaki S. The effect of maternal smoking during pregnancy and post-natal household smoking on dental caries in young children. J Pediatr. 2009;155:410-415. [Crossref], [Google Scholar], [Indexed]
- Heidin CA, Larsson A. The ultrastructure of the gingival epithelium in smoker's melanosis 1984. J Periodontal Res. 1984;19:177-190. [Crossref], [Google Scholar], [Indexed]
- Gilhar A, Pillar T, David M, Eidelman S. Melanocytes and langerhans cells in aged versus young skin before and after transplantation onto nude mice. J Investig Dermatol. 1991;173:47-53. [Crossref], [Google Scholar], [Indexed]
- Sugiyama S, Hanioka T, Naito T. Gingival pigmentation affected by smoking among different age groups: A quantitative analysis of gingival pigmentation using clinical oral photographs. Int J Enviro Res Public Health. 2017;14:E880. [Crossref], [Google Scholar], [Indexed]
- Chen Z, Peto R, Zhou M, Iona A, Smith M, Yang L, et al. Contrasting male and female trends tobacco-attributed mortality in China: Evidence from successive nationwide prospective cohort studies. 2015;386:1447-1456. [Crossref], [Google Scholar]
- Hagifattahi F, Azarshab M, Haghgoo R, Lesan S. Evaluation of the relationship between passive smoking and oral pigmentation in children. J Dent (Tehran). 2010;7:119-23. [Crossref], [Google Scholar], [Indexed]
- Donetti E, Gualerzi A, Bedoni M, Volpari T, Sciarabba M, Tartaglia G, et al. Desmoglein 3 and Keratin 10 expressions are reduced by chronic exposure to cigarette smoke in human keratinized oral mucosa explants. Arch Oral Biol. 2010;55:815-823. [Crossref], [Google Scholar], [Indexed]
- Sridharan S, Ganiger K, Satyanar AA, Rahal A, Shetty S. Effect of environmental tobacco smoke from smoker parents on gingival pigmentation in children and young adults: A cross-sectional study. J Periodontol. 2011;82:956-962. [Crossref], [Google Scholar], [Indexed]
- Nancy AFK, Tamer MAA. Smoking prevalence and its impact on oral health status among a sample of secondary school male students in Giza, Egypt. Institute of African Research and Studies. 2006. [Crossref], [Google Scholar], [Indexed]




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.