Characteristic of the Vincanine Relaxing Action on the Contractive Activity of Aortic Smooth Muscle Cells
2 Department of Veterinary, Nukus Branch of the Samarkand Institute of Veterinary Medicine, Nukus, Uzbekistan
3 Department of Chemistry, Plant Substances, Academy of Sciences of the Republic of Uzbekistan, Tashkent, Uzbekistan
Published: 30-Sep-2021
Citation: Mirzayeva Y , et al. Characteristic of the Vincanine Relaxing Action on the Contractive Activity of Aortic Smooth Muscle Cells. Ann Med Health Sci Res. 2021;11:S4:45-49
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Background & Aim: The special attention is paid to natural biologically active compounds of plant origin, which have a wide range of pharmacological effects and specifically interact with various types of ion channels. The relaxant effect of some diterpenoid alkaloids on the Ca channels of smooth muscle cells has been studied. The aim of this work was to study the mechanism of the relaxant effect of vincanine.
Methods: Isolated preparations of smooth muscle segments of the aorta of white outbred rats (200 g-250 g) were used. The prepared aortic segment was placed into a special experimental chamber with a volume of 5 ml, where it was fixed with a silver wire between the rod of an electromechanical transducer (FT.03, Grass, USA). The contractile activity of the aorta preparations was recorded using a PIIT amplifier (Grass, USA) and an Endim 621.02 recorder (Germany). We used phenylephrine verapamil and ethylene glycol bis-aminoethyl tetra acetate from Sigma (St. Louis, Mo, USA). Results: The indole alkaloid vincanine in a wide concentration range did not affect the basal tone of the rat aorta preparations. The studied alkaloid effectively relaxed the preparations of the aorta previously contracted with hyperpotassium solutions. The relaxing effect of vincanine was dose-dependent, and with an increase in the alkaloid concentration in the range of 10 μM-50 μM, the force of contraction of the rat aorta preparation induced by 50 mM KCl decreased from 15.7%-4.2% to 93.6%- 3.6%. The EC50 value at which vincanine relaxed the aortic preparation by 50% was 16.3 μM. Conclusion: The blockade of the receptor-driven Ca2 + channels may also be involved in providing the relaxant effect of the investigated alkaloid vincanine. The indole alkaloid vincanine has a relaxant activity and relaxes the preparations of the rat aorta, previously contracted with a solution of hyperpotassium and phenylephrine.
Keywords
Aorta; Ion channels; Receptors; Vincanine; KCl; Phenylephrine
Introduction
At present, all over the world there is a wide spread of cardiovascular system pathologies, which occupy leading positions in the overall structure of morbidity and mortality in most countries of the world. [1] One of the example in the development of diseases of the cardiovascular system is arterial hypertension, which is considered the leading risk factor for the development of myocardial infarction and stroke. In this regard, one of the most urgent tasks of modern medicine is the development of new approaches to adequate therapy for arterial hypertension, based on the latest advances in molecular pharmacology. Simultaneously, information on the pharmacological properties of key targets of blood vessels, with which the pathogenesis of arterial hypertension is directly related, is of particular importance. Particular attention is paid to elucidating the mechanisms of modulation of Ca2+ transporting systems of smooth muscles, the dysfunction of which leads to the development of pathological processes in the blood vessels.
In this case, the decisive role is played by violations of the contractile activity of smooth muscle cells, which is regulated by the intracellular level of Ca2+ ions, supported by Ca2+ transport systems of the plasma lemma and sarcoplasmic reticulum. [2] It was found that violations of the contractile activity of smooth muscle cells, observed in arterial hypertension, are directly related to the violation of the function of their Ca2+ transport systems. [3] At the same time, special attention is paid to natural biologically active compounds of plant origin, which have a wide range of pharmacological effects and specifically interact with various types of ion channels. The relaxant effect of some diterpenoid alkaloids on the Ca channels of smooth muscle cells has been studied. [4] Thus, in the regulation of blood pressure, a significant role is assigned to antihypertensive drugs, namely, plant extracts in in vitro experiments on the aorta of rats. [5-7]
Plant indole alkaloids characterized by a wide variety of chemical structures and a wide range of biological effects, so as known information the class of alkaloids containing in their structure the nucleus of indole or its derivatives. More than 4100 indole alkaloids are known. [8] A significant proportion of indole alkaloids also contain isoprenoid structural elements. Many indole alkaloids have physiological activity, some of them are used in medicine.
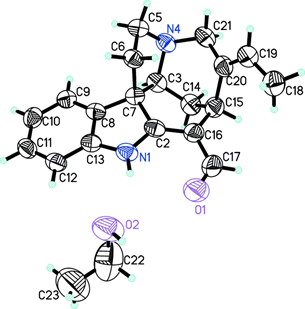
In particular, vincanine [Figure 1] has a pronounced relaxing effect, which is determined by the presence of specific functionally important groups in its structure, has a relaxing effect and relaxes rat aorta preparations, previously reduced by phenylephrine and hyper potassium solutions.
In this regard, the aim of this work was to study the mechanism of the relaxant effect of vincanine. The title compound, C19H20N2O · C2H5OH, is an ethanol solvate of an indole alkaloid that has been extracted from the Vinca erecta plant. The fused piperidine ring assumes the approximate boat conformation, and the pyrrolidine ring assumes the shell conformation with one of the methylene carbon atoms on the valve. The intra molecular hydrogen bond N-HO forms a ring motif S6. In the crystal, the molecules of nor fullorocurarine and ethanol are linked in a chain in the direction of the c axis through hydrogen bonds N-H O and O-H N. [9]
To do scientific work on the all animals care and experimental procedures were approved by the animal experiment committee of the institute of biophysics and biochemistry of the national university of Uzbekistan.
Materials and Methods
In the experiments, isolated preparations of smooth muscle segments of the aorta of white outbred rats (200 g-250 g) were used. The rats were killed by cervical dislocation, the chest was opened, the aorta was removed and placed in krebs saline solution, the following composition (mM): NaCl-120.4; KCI-5; NaHCO3-15.5; NaH2PO4-1.2; MgCl2-1.2; CaCl2-2.5; C6H12O6-11.5; pH 7.4. The aorta was cleaned of adipose and connective tissue and cut into segments in the form of rings 3 mm-4 mm wide. The prepared aortic segment was placed into a special experimental chamber with a volume of 5 ml, where it was fixed with a silver wire between the rod of an electromechanical transducer (FT.03, grass, USA) and the base of the chamber. The experimental chamber was perfused with oxygenated carbogen (95% О2, 5% СО2) krebs solution at a constant temperature of 37˚C. Before the experiment, the aortic segments were pre-stretched with a load of 1 g and washed with saline for 60 minutes to achieve equilibrium. Contractions of the aorta preparations were induced by adding phenylephrine (1 μM) or hyperpotassium solution (KCl, 50 mM) to the experimental chamber by equimolar substitution of NaCl in the krebs solution with it. Calcium-free solutions were also used in the experiments, for which Ca2+ ions were excluded from the krebs solution, and ethylene glycol-bis-aminoethyl-tetraacetate (1 mM) was added to bind their traces. The contractile activity of the aorta preparations was recorded using a PIIT amplifier (grass, USA) and an endim 621.02 recorder (Germany).
We used phenylephrine verapamil and ethylene glycol bis-aminoethyl tetraacetate from Sigma (St. Louis, Mo., USA). Statistical data processing was done by using the Origin Pro 7.5 software (OriginLab Corporation; USA). The amplitude of contractions was expressed in % of the maximum contraction (taken as 100%) induced by phenylephrine or hyper potassium solution and was calculated as the arithmetic mean for 4-5 different experiments. The statistical significance of differences between control and experimental values was determined for the data set using a paired t-test. P-values<0.05 indicate statistically significant differences.
Results
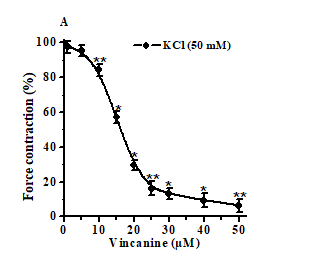
In preliminary experiments, the indole alkaloid vincanine in a wide concentration range did not affect the basal tone of the rat aorta preparations. These data indicate that, at rest, the studied alkaloid does not affect the functional activity of the contractile apparatus of rat aortic smooth muscle cells. However, the studied alkaloid effectively relaxed the preparations of the aorta previously contracted with hyperpotassium solutions, has a pronounced relaxing effect [Figure 2]. The relaxant effect of vincanine was dose-dependent, and with an increase in its concentration, the degree of suppression of contractions of aortic preparations induced by hyper potassium solutions increased markedly. The relaxing effect of vincanine was dose-dependent, and with an increase in the alkaloid concentration in the range of 10 μM-50 μM, the force of contraction of the rat aorta preparation induced by 50 mM KCl decreased from 15.7%-4.2% to 93.6%-3.6% [Figure 2]. The EC50 value at which vincanine relaxed the aortic preparation by 50% was 16.3 μM.
On the ordinate, the force of contraction of the rat aorta preparations, expressed in %, of the control obtained under the influence of 50 mM KCl, was taken as 100%. The abscissa shows the concentration of vincanine (μM). In all cases, P<0.05 (n=6).
Considering that the KCl-induced contraction of the rat aorta is mainly provided by the activation of voltage-dependent Ca2+ channels of the plasmolemma of smooth muscle cells and Ca2+ ions coming through them, [10] it can be assumed that the observed relaxant effect of vinkanine is possibly realized as a result of blocking these channels by it. At the same time, blocking these channels and suppressing the entry of Ca2+ ions into smooth muscle cells, vincanine, apparently, causes a decrease in the intracellular level of Ca2+ ions, which leads to relaxation of the rat aorta preparations. To test this assumption, we studied the dependence of the relaxant action of vinkanine on the concentration of Ca2+ ions in the incubation medium. It is known that in solutions that do not contain Ca2+ ions, hyperpotassium solutions do not cause contractions of aortic preparations, and the cumulative addition of Ca2+ ions under these conditions is accompanied by the development of contractions that reach the control amplitude at 2.5 mM CaCl2. [10,11]
In particular, in the presence of 50 μM vincanine, the addition of 2.5 mM CaCl2 to the calcium-free solution caused a contraction of the aorta preparation, which was 81.9% ± 4.2% less than the contraction recorded in the absence of the alkaloid. In these experiments, an increase in the concentration of CaCl2 (0 mM-2.5 mM) in the incubation medium was accompanied by a stepwise increase in the force of contraction of the aorta due to the penetration of Ca2+ ions through the L-type Ca2+ channels. In the presence of the studied alkaloid in the incubation medium, the development of contractions in response to an increase in CaCl2 was noticeably suppressed. The results of these experiments show that the relaxant effect of vincanine is associated with a decrease in the level of (Ca2+) ions in smooth muscle cells, which, apparently, occurs as a result of suppression of their entry through the L-type Ca2+ channels.
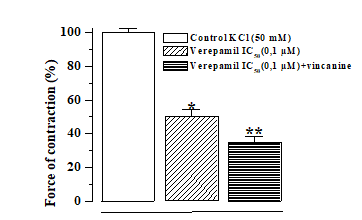
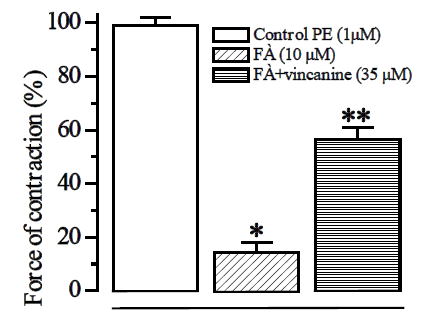
To confirm the interaction of the studied alkaloid with voltage-dependent Ca2+ channels in the plasma lemma of smooth muscle cells, we performed experiments with a specific blocker of these channels, verapamil. [12] As shown by the results of these experiments, verapamil itself effectively relaxes the rat aorta preparations, previously contracted with hyper potassium solutions, by suppressing the influx of Ca2+ ions into the smooth muscle cells of the rat aorta. In these experiments, it was found that in the presence of a 0.1 μM verapamil concentration corresponding to its IC50 value, the addition of vincanine (15.9 μM) to the incubation medium further relaxed the rat aorta preparations [Figure 3].
Discussion
It follows from the results obtained that the blockade of L-type Ca2+ channels may underlie the relaxant action of the alkaloid vincanine. However, the retention of the relaxant effect of the studied alkaloid in the presence of verapamil indicates that, in addition to the blockade of L-type Ca2+ channels, other mechanisms of Ca2+ transport into smooth muscle cells can also be involved in its provision.
Ca2+ channels of the sarcoplasmic reticulum are a structure that, in response to depolarization of plasma membranes, provides a rapid massive release of Ca2+ from the reticulum into the cytoplasm. The released Ca2+ binds to intracellular regulatory Ca-binding proteins, which leads to the activation or inhibition of various biochemical reactions (more about Ca2+ binding proteins and their functions. [13,14] In smooth muscle cells, Ca2+ binds to calmodulin, which leads to actomyosin activation ATPase for relaxation, it is necessary to remove Ca2+ from the cytoplasm, again accumulating it in the cavities of the sarcoplasmic reticulum for reuse in subsequent cycles of contraction-relaxation or its removal from the cell into the environment. In this regard, in order to further characterize the mechanism of the relaxant action of vincanine, we studied its effect on the contraction of rat aorta preparations induced by the α-adrenergic receptor agonist phenylephrine, the development of which is provided by Ca2+ entering smooth muscle cells through receptor-gated Ca2+ channels.
Ca2+ ions enter smooth muscle cells not only through Ca2+ dependent channels, but also through channels controlled by Ca2+ receptors, and are released from the sarcoplasmic reticulum. In muscles, calcium release is triggered in different ways, mainly via ryanodine receptors. In smooth muscle, an electrical impulse triggers the release of calcium into the cell through L-type calcium channels located in the cell membrane. Calcium ions bind to ryanodine receptors and activate them, as a result of which the level of calcium in the cell rises rapidly. [15] An important role in the regulation of Ca2+ homeostasis in smooth muscle cells, along with voltage-gated L-type Ca2+ channels, is also played by receptor-gated Ca2+ channels, which are activated upon stimulation of α-adrenergic receptors.
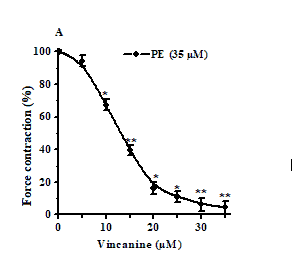
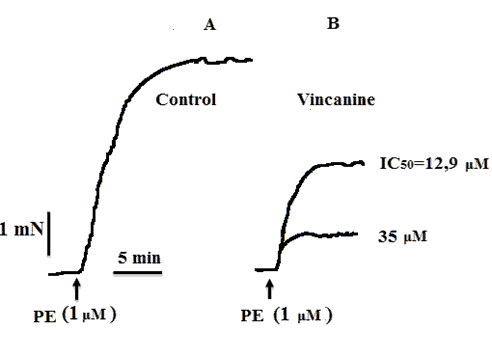
In these studies, we found that the alkaloid vincanine also effectively relaxes rat aortic preparations under conditions of phenylephrine-induced contracture. At the same time, the relaxant effect of vincanine was dose-dependent, and at its concentration of 3 μM, it caused relaxation of the aorta preparation by 18.2% ± 4.4%, and maximum relaxation up to 95.6% ± 3.8% was observed at its concentration. Concentration 35 µM [Figures 4 and 5]. In this regard, to assess the effect of the studied alkaloid on receptor-gated Ca2+ channels, we studied their effects on the contractions of aortic preparations induced by the α-adrenergic receptor agonist phenylephrine. Phenylephrine induces aortic contractions, which, in the presence of verapamil, are mainly provided by Ca2+ ions entering smooth muscle cells through receptor-gated Ca2+ channels. The EC50 value of vincanin under these conditions was 12.9 μM. In additional experiments, phenylephrine (1 μM) in the presence of verapamil (0.1 μM) induced contractions of the aorta, the force of which was 10.9% ± 2.3% less than the force of contractions caused by it in the absence of verapamil. The addition of the alkaloid vincanine under these conditions led to a significant suppression of the strength of phenylephrine-induced contractions of the aorta, which decreased by 65.4% ± 4% from the control, respectively.
Figure 5: Influence of the alkaloid vincanine on rat aortic contractions induced by phenylephrine in Krebs solutions not containing Ca2+ ions. Records of the alkaloid effects on aortic contractions induced by phenylephrine (1 μM) in Krebs solutions without Ca2+ ions. A: Control, B: In the presence of 12.9 μM and 35 μM vincanine.
At the same time, the relaxant effect of vincanine under the conditions of PE-induced contracture was also observed in calcium-free solutions, the development of which is mainly provided by the Ca2+ ions released from the SR. As can be seen in Figure 5, when Ca2+ ions are excluded from the incubation medium, phenylephrine causes a contraction of the rat aorta preparation, which is 70.1 ± 3.5% less than the contraction of induced phenylephrine in the presence of Ca2+ ions. The addition of 12.9 μM vincanine under these conditions led to an additional decrease in the amplitude of contraction by 46.2% ± 4%. The results of these experiments indicate that the relaxant effect of vincanine, under conditions of phenylephrine-induced contracture, is mainly due to its effect on the transport of Ca2+ ions through the receptor-driven Ca2+ channels of the plasmalemma and their release from the sarcoplasmic reticulum of smooth muscle cells.
The results of these experiments show that the relaxant effect of the alkaloid under conditions of contracture caused by phenylephrine is mainly associated with their effect on the transport of Ca2+ ions through the receptor-driven Ca2+ channels of the plasmalemma and their release from the sarcoplasmic reticulum of smooth muscle cells. The IC50 values obtained for vincanin were 12.9 μM, respectively. This is confirmed by experiments with a blocker of α-adrenergic receptors, phentolamine, in the presence of which the effect of the alkaloid on PE-induced contractions of the aorta decreased by 43.4% ± 4.1%, respectively, from the control obtained in the absence of phentolamine [Figure 6]. These results indicate that the blockade of receptor-gated Ca2+ channels may also be involved in providing the relaxant effect of the studied alkaloid.
These results indicate that the blockade of the receptor-driven Ca2+ channels may also be involved in providing the relaxant effect of the investigated alkaloid vincanine. Thus, the analysis of the obtained data showed that the indole alkaloid vincanine has a relaxant activity and relaxes the preparations of the rat aorta, previously contracted with a solution of hyperpotassium and phenylephrine.
At the same time, the relaxing effect of vincanine was more pronounced and was dose-dependent; the relaxant effect of vincanine was preserved in the presence of verapamil, and it equally effectively relaxed the rat aorta preparations under conditions of contracture induced by KCl and phenylephrine.
Conclusion
The achieved data indicate that many mechanisms are involved in the implementation of the relaxant action of vincanine, including blockade of voltage-dependent Ca2+ channels (under conditions of contracture induced by KCl), suppression of Ca2+ ion transport through channels controlled by Ca2+ receptors, and their release from the sarcoplasmic reticulum (under conditions contracture caused by phenylephrine).
According to results of the research there was no doubt that these features of the relaxing action of vinkanine are due to the presence in its structure.
Acknowledgements
This work was supported by the grant FT-O-2021-154 of the coordinating committee for the development of science and technology under the Cabinet of Ministers of the Republic of Uzbekistan.
Conflict of Interest
Each author declares that has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article.
References
- Cardiovascular diseases-monitoring and control. Scitech Europa. 2018;27.
- Jackson WF. Ion channels and vascular tone. Hypertension. 2000;35:173-178.
- Mitchell BM, Chitaley KC, Webb RC. Vascular smooth muscle contraction and relaxation. In: Hypertension primer: The essentials of high blood pressure, edited by Izzo jl and black hr. Am Heart Assoc. 2003;pp:97-99.
- Mirzayeva YuT, Usmanov PB, Omonturdiyev SZ, Ibragimov EB, Abrayeva ZCh. Characteristic of the vasorlaxant action of the talatisamine alkaloid and its derivatives Journal of Pharmaceutics and Pharmacology Research. 2021;pp:1-5.
- Lee KJ, Jung GY, Ham IYB, Kim H, Choi HY, Endothelium-independent vaso relaxation effects of sigesbeckia glabrescens (Makino) makino on isolated rat thoracic aorta. Phytother Res. 2013;27:1308-1312.
- Park JY, Shin HK, Lee YJ, Choi YW, Bae SS, Kim CD. The mechanism of vaso relaxation induced by Schisandra chinensis extract in rat thoracic aorta. J Ethnopharmacol. 2009;121:69-73.
- Koh SB, Kang MH, Kim TS, Park HW, Park CG, Seong YH, et al. Endothelium-dependent vasodilatory and hypotensive effects of Crotalaria sessiliflora L. in rats. Biol Pharm Bull. 2007;30:48-53.
- David SS. Plant secondary metabolism. Springer. 2001:628-776.
- Adizov SM, Ashurov J. Norfluoro-curarine ethanol monosolvate. Acta Crystallogr Sect E Struct Rep Online. 2013;9:1100.
- Ratz P, Berg K,Urban N, Miner A. Regulation of smooth muscle calcium sensitivity: KCl as a calcium-sensitizing stimulus. Am J Physiol Cell Physiol. 2005;288: 769-783.
- Nishimura K, Ota M, Ito K. Existence of two components in the tonic contraction of rat aorta mediated by α1-adrenoceptor Br J Pharmacol. 2002;102:215-221.
- Сleary L, Vandeputte C, Kelly JG, Docherty JR. Actions of R and S verapamil and nifedipine on rat vascular and intestinal smooth muscle. Auton Autacoid Pharmacol. 2004;24:63-67.
- Gusev NB. Intracellular Ca-binding proteins. Part 1. Classification and structure. Soros Educ J. 1998;5:2-9.
- McFadzean I, Gibson The developing relationship between receptor-operated and store-operated calcium channels in smooth muscle. Br J Pharmacol. 2002;35:1-13.
- Wier WG, Morgan KG. Alpha-adrenergic signaling mechanism in a contraction of resistance arteries. Rev Physiol Biochem Pharmacol. 2003;150:91-139.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.