Characteristics of Collagen to Differentiate Fibrous Hyperplasia from Mesenchymal and Mixed Odontogenic Tumors: A Polarized Microscopic Study
2 Division of Oral & Maxillofacial Pathology, Sree Anjaneya Institute of Dental Sciences, Calicut, Kerala, India
3 Department of Computer and information Science, Annamalai University, Chidambaram, Tamil Nadu, India
Citation: Mohamed Nassar M. Characteristics of Collagen to Differentiate Fibrous Hyperplasia from Mesenchymal and Mixed Odontogenic Tumors: A Polarized Microscopic Study. Ann Med Health Sci Res. 2017; 7: 319-324
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Context: Mesenchymal odontogenic tumors arise from the remnants of the mesenchymal portion of tooth germ. Central odontogenic fibroma (COF), odontogenic myxoma (OM) and Ameloblastic fibroma (AF) are closely related and show varied clinical presentation and overlapping histopathology. They are differentiated by their collagen quantity, maturity and distribution. Diagnostic difficulties are posed when compared with enlarged dental follicle (EDF) as it closely resembles them. Aim: To analyze the collagen in COF, OM, AF and EDF both quantitatively and qualitatively to choose the best attributes which characterize them. Settings and Design: Polarized microscopy and picro-sirius red staining to visualize every collagen bundle in birefringent state was employed. Material and Methods: 22 tissue sections of EDF, COF, OM and AF were studied for mean collagen bundles in 100 μm² areas, the maturity of 50 bundles each of thin, thick and intermediate sizes and the number of mature, immature and intermediately mature bundles in 100 μm² irrespective of thickness. Statistical analysis used: ANOVA, Tukey and Scheffe test were used to find the significance. Results: COF showed maximum number of collagen bundles in 100 μm² area (P value<0.001). For every 50 intermediately thick bundles, the most red were EDF 20(3); the most green were COF 28(7) and the most yellow were OM 23(4). For every 50 thick bundles, the most red were OM 31(5) and the most green were COF 33(5). Conclusions: Among these lesions maturity of intermediate and thick bundles varied significantly. We conclude that the study of thick fibres is best suited to differentiate them.
Keywords
Odontogenic tumors; Ectomesenchyme; Collagen bundles
Introduction
Mesenchymal odontogenic tumors account for as high as 8.3% to as low as 3.8% of all the odontogenic tumors [1-3]. These tumors resemble their tissue of origin by morphology and function. The WHO classification of tumors of the head and neck classifies them as tumors of mesenchyme and/or odontogenic ectomesenchyme with or without odontogenic epithelium and include odontogenic fibroma, odontogenic myxoma/myxofibroma and cementoblastoma [4].
Central odontogenic fibroma (COF) is an uncommon neoplasm accounting for 0.2% to 2.1% of the odontogenic tumors [1-3]. They show considerable histological diversity and according to Gardner, two types of COF, the “simple type” and the “complex type” have been reported. The simple type is relatively acellular with fibres that are delicate and interspersed with abundant ground substance. In contrast, the complex type of COF is more cellular and contains fibroblastic strands that are interwoven with less cellular areas. The odontogenic rests are said to be present many times. The simple type is said to have originated from the dental follicle and the complex type from the periodontal ligament [5]. Calcified material such as dysplastic cementum, osteoid or dysplastic dentin may also be found. The WHO classification designates these two variants as epitheliumpoor type and epithelium-rich type respectively [4]. However, there does not seem to be a universal acceptance of this division into two types [3].
Odontogenic myxoma (OM) is a tumour of primitive connective tissue with a frequency of 2.2% to 4.6% among the odontogenic tumors [1-3]. This is the most common tumor of heart in adults and can occur in other locations also. The tumour is made up of scattered ropy collagen bundles haphazardly arranged. The stroma is loose with abundant mucoid ground substance. The cells are stellate, spindle and/or round with long fine anastamosing processes. The fibroblast which produces this tissue is suggested to be of myofibroblastic origin [6]. When the collagen fibres are more, they are named fibromyxomas or myxofibromas [4].
Among the mixed odontogenic tumors, ameloblastic fibroma (AF) may sometimes be a histological mimic to OM. AF accounts for 1.2% to 2% of odontogenic tumors [1-3]. Microscopically, they show bilaminar strands of epithelium and myxomatous stroma with a few delicate collagen bundles and very much resemble the odontogenic ectomesenchyme. There are studies which divide this lesion into neoplastic type and pericoronal hamartomatous type [7].
Dental follicle is a normal developmental structure that appears as thin, semicircular radiolucency around unerupted or impacted teeth. Sometimes, enlarged dental follicles (EDF) mimic a pathosis. In a large study of dental follicular tissues by Kim and Ellis, it was found to show collagenous connective tissue, myxoid areas and confirmed dental papillae-like areas [8]. The ratio of non-pathologic to pathologic lesions in the peri-coronal tissues of impacted teeth increases with age [9].
The tumors OM, COF and AF, at times, show overlapping histopathologic features compared with EDF. Owing to the fact that they all have their origin from the ectomesenchyme and all these tumors and EDF may show foci of calcifications and remnants of odontogenic epithelium, and the fact that in fair number of cases all of them show association with the crowns of unerupted teeth, we thought that a comparison of their fibre characteristics with EDF would shed some light to differentiate them.
Therefore the present study is aimed to analyze the characteristics of the collagen fibres of the, COF, OM, AF and EDF which dictates the behaviour of these mesenchymal lesions. A quantitative and qualitative evaluation of collagen using modified picrosirius-red staining and polarizing light microscopy was employed to find which collagen characteristic can be employed best to differentiate these lesions.
Subjects and Methods
The retrospective study was conducted after obtaining institutional ethical committee approval and included 22 cases of COF, OM, AF and EDFs, from the archival tissues of the Department of Oral and Maxillofacial Pathology. Out of these, 6 cases were of OM, 5 cases were of AF, 3 cases of COF and 8 cases of EDF. The diagnoses of the lesions were based on clinical description, radiographic evidence and histopathologic features, confirmed by two independent oral pathologists.
Thin paraffin sections of 5μ were prepared, de-parafinized, hydrated in descending grades of alcohol and taken to distilled water; incubated for 1 hour in 0.1% (w/v) Sirius Red F3B (CI 35780) in saturated picric acid solution; differentiated in 1% glacial acetic acid for 30minutes; rinsed in distilled water, counterstained with Meyer’s hematoxyllin and mounted.
Picrosirius red stained sections were examined under polarizing microscope using a circular polarized light which aids in visualizing maximum amount of collagen fibres. Photomicrographs were made in 40X magnification in five representative fields for each sample. The collage fibres were assessed for number, color and thickness. The counting of collagen bundles and measuring of bundle thickness were performed using “Image J” software (Image J 1.41°, Wayne Rasband, National Institute of Health, USA) after standardization. Polarization colors were determined separately for thin fibres (<0.8 μm), intermediate fibres (0.8-1.6 μm) and thick fibres (1.6-2.4 μm) [10]. Polarization colors of the collagen bundles were observed to be red to reddish orange, green to greenish yellow and yellow to yellowish orange. For this study we recorded red and reddish orange as “red”, green and greenish yellow as “green” and yellow and yellowish orange as “yellow”.
The collagen fibres were assessed as follows:
• The total number of fibre bundles in every 100 μ² area was counted after laying a grid of that size. Three such areas were counted in each photomicrograph. This was performed for all the five photomicrographs of each section and the mean was calculated for each sample.
• 50 fibres bundles of thin, thick and intermediate fibres were identified in each photomicrograph and the color emitted by these fibres was recorded. Five such areas were counted per sample and the results were expressed as average per high power field out of five fields. The colors observed were recorded by three independent observers.
• The number of mature, immature and intermediate fibre bundles showing red, green and yellow colors respectively were counted in a 100 μ² area irrespective of the bundle size.10 Three such areas were counted in each photomicrograph and the mean was calculated for each sample.
The data were analyzed Using standard ANOVA analysis, Tukey and Scheffe tests for statistical significance among the different lesions. A P value of<0.05 was considered statistically significant. The Statistical Package for Social Sciences Software (SPSS Inc., Version 22.0 Armonk, NY: IBM Corp.) was used for data analysis.
Results
Number of fibres in 100 μ² area.
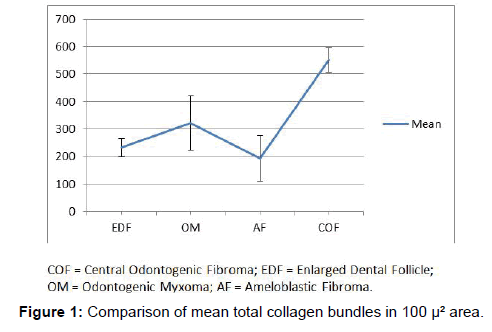
the first part of the study, all the fibres present in 100 μ² area were counted. COF showed the maximum number of fibres and AF the least among the odontogenic tumors whereas, the OM was significantly higher than AF (P value<0.05) and was in between AF and COF (P value<0.001). COF collagen bundles were very significantly higher than EDF (P value<0.001). However, there was no significant difference between EDF and the other two. The results showed that there was significant difference among all the groups studied (Figure 1 and Table 1).
|
Tukey |
Mean Difference | Std. Error | P value |
|---|---|---|---|
| COF vs.. EDF | 318.46 | 47.49 | (<0.001) |
| COF vs.. OM | 229.5 | 49.61 | -0.001 |
| COF vs. AF | 358.06 | 51.23 | (<0.001) |
| OM vs. AF | 128.56 | 42.48 | -0.033 |
|
Scheffe |
|||
| COF vs. EDF | 318.46 | 47.49 | (<0.001) |
| COF vs. OM | 229.5 | 49.61 | (<0.01) |
| COF vs. AF | 358.06 | 51.23 | (<0.001) |
COF = Central Odontogenic Fibroma EDF = Enlarged Dental Follicle OM = Odontogenic Myxoma AF = Ameloblastic Fibroma
Table 1: Comparison of total number of fibres in 100 µ2 area between COF, OM, AF and EDF
Color emitted by different thickness of fibres
The second part of the study is to compare the color emitted by the fibres according to the thickness. Thin, thick and intermediate collagen bundles were studied for the color emission. On analyzing the color emitted by the thin fibres among the four lesions, there was no significant difference between the groups.
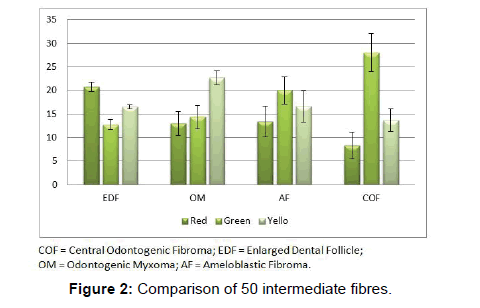
Comparison of intermediate fibres showed significant difference between groups. Red color emitted by the intermediate fibres of the EDF were significantly more than those of COF (P<0.05). Green color emitted by the intermediate fibres of COF were significantly more than EDF and OM (P<0.05). Yellow color emitted by the intermediate fibres of OM were significantly more than COF (P<0.05). (Figure 2 and Table 2).
| Comparison of 50 intermediate sized collagen bundles of COF, OM, AF and EDF based on colour | |||
|---|---|---|---|
| Colour | Mean Difference | Std. Error | P value |
| Red | |||
| COF vs. EDF | -12.42 | 3.59 | -0.014 |
| Green | |||
| COF vs. EDF | 15.25 | 3.65 | (<0.01) |
| COF vs. OM | 13.66 | 3.81 | -0.01 |
| Yellow | |||
| COF vs. OM | -9 | 3.06 | -0.04 |
| Comparison of 50 thick sized collagen bundles of COF, OM, AF and EDF based on colour | |||
| Red | |||
| COF vs. EDF | -28.08 | 3.05 | (<0.001) |
| COF vs. AF | -9.53 | 3.29 | -0.044 |
| OM vs. AF EDF vs. AF OM vs. COF |
14.3 18.55 23.83 |
2.73 2.57 3.19 |
(<0.001) |
| Green | |||
| COF vs. EDF COF vs. OM OM vs. AF EDF vs. AF |
28.83 29.66 -21.33 -20.5 |
2.76 2.88 2.47 2.32 |
(<0.001) |
| Yellow | Not significant | ||
COF = Central Odontogenic Fibroma EDF = Enlarged Dental Follicle OM = Odontogenic Myxoma AF = Ameloblastic Fibroma.
Table 2: Comparison of 50 intermediate and thick sized collagen bundles of COF, OM, AF and EDF based on colour
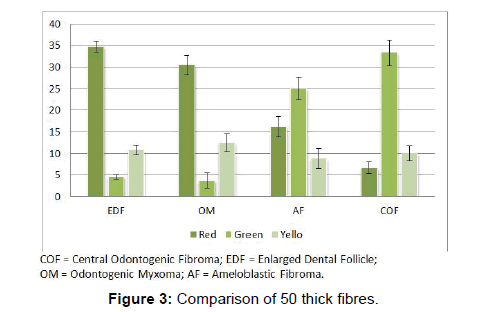
Comparison of thick fibres emitting red colors were highly significant among the four lesions (P<0.01). Thick fibres of EDF showed the maximum red color (34.8 ± 1.3) and that of COF showed the least (6.7 ± 1.3). Thick fibres of OM were very significantly red when compared to COF and AF (P<0.001). The thick fibres of EDF were more significantly red than AF and COF (P<0.001).
The difference between thick fibres which emit green color were significantly different among the four lesions studied (p Level<0.01). Most number of thick fibres were green in COF and least number of thick fibres were green in OM (P<0.001). Thick fibres of COF emitted green color which was statistically significant than EDF (P<0.001). Analysis of fibres of AF showed them very significantly greener than EDF (P<0.001) and OM (P<0.001) (Figure 3 and Table 2).
Thick fibres emitting yellow color showed no significant difference between the lesions.
The number of fibre bundles based on color alone
The number of collagen bundles of mature, immature and moderately mature fibres showing red, green and yellow color respectively was counted irrespective of their thickness in 100 μ² area. There appeared no significant difference between the four lesions studied.
Discussion
Differentiating the fibrous non-neoplastic lesions around unerupted crowns from odontogenic tumors pose diagnostic difficulties. Many of the histological findings of EDF mimic the features found in other fibrous odontogenic tumors [9]. AF being similar to the dental papilla and dental follicle, chances of diagnosing a dental follicular tissue with less dense fibre content as AF cannot be denied. Myxoid changes in an EDF sometimes are mistaken for an OM. The dental follicular tissue can contain interlacing fibrous bundles and some of the residues of odontogenic epithelium and calcifications resembling COF. Report of incisional biopsy as odontogenic fibroma due to non-inclusion of myxomatous areas and final diagnosis as OM is also published [6]. Research shows that a third of the soft tissue associated with 100 impacted molars which showed no radiographic evidence of abnormal pathology were odontogenic cysts or tumors [10,11].
Delineating them possibly at the early stages is difficult and at times, requires histo-chemical technique other than the routine H&E. Conventional connective tissue stains such as Van Gieson and Masson’s trichrome were not used because they depend on differential binding of the combined anionic dyes to tissue components which lack precise selectivity [12]. Sirius red, being an anionic dye, the sulphonic acid groups react with the basic group present in the collagen molecule. Picric acid prevents indiscriminate staining of non-collagenous structures [13]. Collagen fibres are bi-refringent under polarized light. Picrosirius red enhances the birefringence of the collagen. Polarized light system with dark field illumination of collagen suited better for viewing even thin collagen fibres compared to bright field illumination [12]. The collagen quality, organization and packaging is different in different lesions which account for the difference in biologic behaviour of these lesions [14].
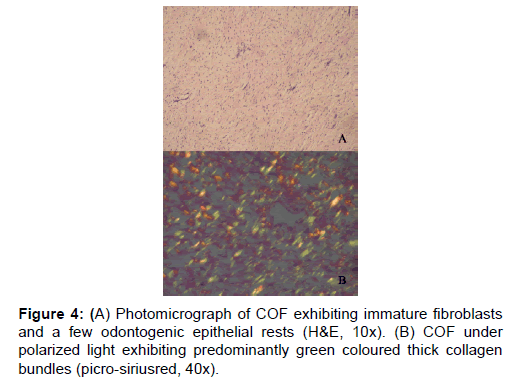
In the earlier studies about the COF, some authors stated that it arises from partially induced somatic mesenchyme that may otherwise develop into periodontal ligament [15]. There is a very good similarity between the COF and the histopathology of the dental follicle. Some investigators have suggested that this lesion, when it has less collagen actually belongs to the spectrum of Odontogenic myxoma and should be designated as myxofibroma [16]. Our study shows that the number of fibres in 100 μm2 area of COF is very significantly higher than EDF (P<0.001). This shows that the COF is the most fibrous counterpart of dental follicle. In the second part of our study it was found that that the thick collagen bundles of EDF and OM were significantly more red than COF (P<0.001) (Figure 1). In addition to this, it was found that the thick fibres of COF were very significantly greener than EDF and OM (P<0.001) (Figure 3). A predominance of thick collagen bundles showing green color can be seen in Figure 4. This shows that as the fibre thickness increases, in COF the maturation process is not normal. These represent pro-collagen, hence do not have the proper packaging of fibres and therefore appear green.10 Observation in dermis is that the pro-collagen has very short half-life and is easily degraded [17]. It is a well-known that COF is a slow growing lesion, present for a long time till diagnosed. Even then, the thick collagen bundles observed here are of the immature type. The increase in the fibroblast population, when correlated with this can be inferred as being in a state of continuous neocollagenesis along with failure of maturation. Any case of COF can show features of complex and simple type in the same lesion. Adalberto et al. in their multi-centric study on COF had common view in this [3]. Therefore, in the present study the COF samples were not sub-typed.
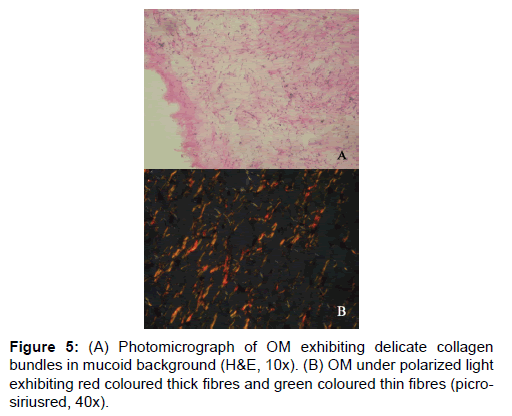
In the studies pertaining to OM, some authors associate its origin with a myxomatous change of an odontogenic fibroma [18]. An EDF showing myxoid changes may also be microscopically similar to myxoma. In these circumstances, studies related to the fibre content of these lesions may help to delineate them. It was noted that the number of fibres in OM was significantly lesser than COF and higher than AF (Table 1 and Figure 1). When studying the intermediately thick fibres, the yellow color fibres of OM out-numbered COF. On the other hand the green color intermediate fibres of OM were lesser than COF (Table 2 and Figure 2). Figure 5 shows predominance of yellow and red color thick bundles and green color thin bundles. This implies that as the fibre bundles increase in thickness in OM the maturation process is undisturbed compared to COF. The paucity of green color in OM compared to COF shows that the immaturity of collagen bundles seen in COF is not the case in OM. No significant difference between EDF and OM shows that both show a normal maturative process for the specified fibre thickness.
The thick bundles of OM were very significantly red than COF and AF. Conversely, the thick bundles of OM were very significantly less green than COF and AF (Table 2 and Figure 3). This reflects that the fibre bundles of OM as they become thicker show good packaging (mature) and least pro-collagen when compared to COF and AF. The diameter of the collagen fibre plays a major role in determining the coloring and intensity of the picrosirius red staining. But it is not only the thickness of the fibre but also the packaging of the collagen fibrils which determines the birefringent color to be red for mature, green for immature and yellow for intermediately mature collagen bundles [13].
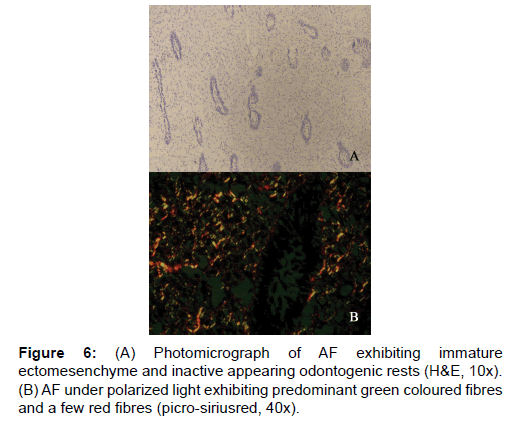
On analyzing the fibres of AF, the results of the present study revealed the total number of fibres in a 100 μm2 area to be the least among the four lesions. This goes well with the already established findings that they contain a few collagen fibres in a primitive ectomesenchyme [7]. The total fibres of AF were very significantly less compared to COF and OM (Table 1 and Figure 1). Even though comparatively AF fibres are least in number among the four lesions studied, it was possible to find thick and intermediate fibre bundles also. When the color emitted by the thick fibres were compared, thick red fibres of AF were significantly greater than COF and very significantly fewer than OM and EDF (Table 2 and Figure 3). This shows that compared to COF the fibre maturity is better in AF and compared to OM and EDF, the fibres of AF are very much immature. The thick green fibres of AF were significantly greater than OM and EDF (Table 2 and Figure 3). Figure 6 shows predominance of green collagen bundles admixed with a few red bundles in AF. It can be inferred that among the three pathologic entities of COF, AF and OM, thick fibres of COF are the most immature, OM being the most mature and AF lies in between these two.
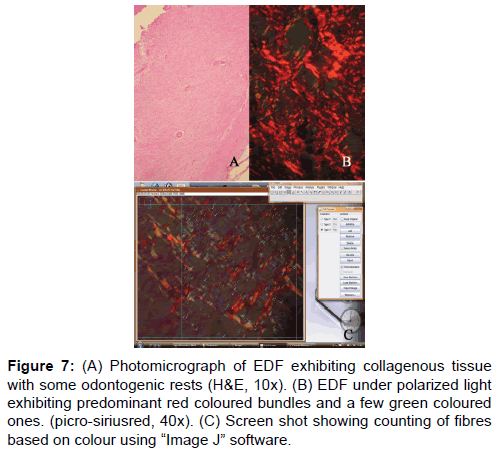
In the third part of the study, the number of red, green and yellow color bundles denoting mature, immature and intermediately mature collagen respectively was counted in a 100 μm² area in all the four lesions. This was done irrespective of the bundle size. Figure 7 shows counting of collagen bundles after placing a grid of 100 μ² area. There was no significant difference between any of these lesions studied. Hence, it can be inferred from this that only when the collagen bundles are studied by their color in correlation with the bundle thickness, there exist significant difference between lesions. Only observing the predominance of color without correlation of fibre thickness does not yield any significance which could help to differentiate them.
Figure 7: (A) Photomicrograph of EDF exhibiting collagenous tissue with some odontogenic rests (H&E, 10x). (B) EDF under polarized light exhibiting predominant red coloured bundles and a few green coloured ones. (picro-siriusred, 40x). (C) Screen shot showing counting of fibres based on colour using “Image J†software.
Conclusion
The study shows that there is a clear difference in the fibre characteristics between the EDF and the tumoral collagen fibres of COF, AF and OM. COF proves to be the most fibrous among the four lesions. Based on the fibre number in a given lesion there is a clear difference among the four lesions studied. The fibre maturity correlated with the bundle thickness shows better significance among the four lesions. EDF shows significant red predominance in intermediate and thick fibre bundles. This shows that as the fibres increase in thickness, there is a proper pattern of maturity. The next lesion which shows maturity correlating to the thickness is OM, although not as equal as EDF. Conversely, in COF and AF there is predominance of immature green and yellow fibres, and as the fibres increase in thickness, there is a variable pattern of maturity. A defect in the packaging of collagen underlies this difference in maturity. The fibre characteristic which is considered as the best parameter to differentiate these lesions is observing thick fibre bundles emitting red color. These findings are more likely to guide the pathologist in accurate diagnosis and hence would have a significant impact in the treatment of the patients.
The laborious procedures of counting collagen bundles and measuring their thickness are the limitations of the study. Computer software eased these works. The study used “image j” software for counting of collagen bundles and thickness. Although they were verified by two independent observers, the numbers obtained are not accurate. So, the study can be considered a semi quantitative study. The site of sample extraction is not standardized. The most representative area of the lesion is used for analysis. This can be considered a limitation of the study.
In the future, customized software may be developed with inclusion of other collagenous lesions and bigger sample sizes which can analyze the collagen fibres and assist in delineating these lesions based on the polarized microscopic pictures.
These findings can serve as an adjuvant in the differentiation of mesenchymal tumors from hyperplastic collagenous tissue.
Acknowledgement
We wish to acknowledge the help of Dr. Jamal Abdul Nasser and Dr. Einstein T Bertin for their help in providing tissue sections for the study. The research is self-funded.
Conflict of Interest
All authors disclose that there was no conflict of interest.
REFERENCES
- Servato JPS, Prieto-Oliveira P, De Faria PR, Loyola AM, Cardoso SV: Odontogenic tumors: 240 cases diagnosed over 31 years at a Brazilian university and a review of international literature. Int J Oral Maxillofac Surg 2013; 42: 288–293.
- Gupta B, Ponniah I. The pattern of odontogenic tumors in a government teaching hospital in the southern Indian state of Tamil Nadu. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2010; 110:32–9.
- Adalberto MT, Guillermo MM, Roman CB, Pablo AV, Victor TR, Ana María CV. Central odontogenic fibroma: New findings and report of a multicentric collaborative study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2011; 112:349 58.
- World Health Organization Classification of tumours. Pathology and genetics of head and neck tumours. IARC Press: Lyon 2005; 284.
- Gardner DG, Central odontogenic fibroma current concept. J Oral Pathol Med 1996; 25:556-561.
- Sivakumar G, Kavitha B, Sarasathi TR, Sivapathasundaram B. Odontogenic myxoma of the maxilla. Indian J Dent Res 2008; 19: 62-65.
- Buchner A, Vered M. Ameloblastic fibroma: A stage in the development of a hamartomatous odontoma. Critical analysis of previously reported cases plus 10 new cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2013; 116:598-606.
- Kim J, Ellis GL. Dental follicular tissue: Misinterpretation as odontogenic tumors. J Oral Maxillofac Surg 1993 Jul; 51: 762-67.
- Curran AE, Damm DD, Drummond JF. Pathologically significant pericoronal lesions in adults: Histopathologic evaluation. J Oral Maxillofac Surg 2002; 60: 613-17.
- Hirschberg A, Buchner A. The central odontogenic fibroma and the hyperplastic dental follicle: study with picrosirius red and polarizing microscopy. J Oral Pathol Med 1996; 25: 125-127.
- Aldesperger J, Campbell JH, Coates DB. Early soft tissue pathosis associated with impacted third molars without pericoronal radiolucency. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2000; 89: 402.
- El-Zahraa F, El-Deen SY, Ezz El-Daela R, Abdel Kerim M. Picrosirius red staining assessment of collagen after dermal roller application: A minimally invasive percutaneous collagen induction therapy. Indian J Dermatopathol Diagn Dermatol 2014;1: 68-74
- Junqueira LCU, Bignolas G, Brentani RR. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J 1979; 11: 447-55.
- Singh HP, Shetty DC, Wadhwan V. Role of collagen fibers in odontogenic cysts. Natl J of Maxillofac Surg 2012; 3: 15-20.
- Marx RE, Stern D. Oral and maxillofacial pathology: A rationale for diagnosis and treatment, Second edition. 2012 Quintessence Publishing co. Inc.
- Noffke CE, Raubenheimer EJ, Chabikuli NJ.. Odontogenic myxoma: A review of the literature and review of 30 cases from south Africa. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2007;104: 101-109
- Ilja L. Kruglikov NE. Collagenesis in non-invasive aesthetic treatments. Journal of Cosmetics, Dermatological Sciences and Applications, 2013, 3, 1-5
- Peter A. Reichart Hans P. Philipsen O. Odontogenic tumors and Allied lesions. Quintessence Publishing Co. Ltd., London. 2004. 183.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.