Comparative Evaluation of Salivary IgA, Flow Rate, pH and Antioxidant Capacity in Children with β-Thalassemia and Healthy Children
Received: 07-Aug-2024, Manuscript No. Amhsr-24-144810; Editor assigned: 09-Aug-2024, Pre QC No. Amhsr-24-144810 (PQ); Reviewed: 28-Aug-2024 QC No. Amhsr-24-144810; Revised: 06-Sep-2024, Manuscript No. Amhsr-24-144810 (R); Published: 16-Sep-2024
Citation: Velagala D, et al. Comparative Evaluation of Salivary IgA, Flow Rate, pH and Antioxidant Capacity in Children with β-Thalassemia and Healthy Children. Ann Med Health Sci Res. 2024;14:1044-1048
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Thalassemia is a prevalent genetic hemoglobin disorder, particularly significant in South East Asia and Africa, with India reporting a prevalence of approximately 3.3%. Children with betathalassemia major require regular blood transfusions, which lead to complications, including iron overload and damage to various organs, including salivary glands. This study examines salivary parameters, including secretory immunoglobulin A (sIgA), pH, buffer capacity, flow rate and total antioxidant capacity, in children aged 5-12 years diagnosed with beta-thalassemia major, compared to healthy controls. A total of 40 children with beta-thalassemia and 40 healthy age- and sex-matched controls were studied. Results indicated significantly lower mean levels of salivary IgA (156.07 ± 30.27 vs. 184.20 ± 33.25), pH (6.42 ± 0.17 vs. 7.00 ± 0.13), buffer capacity (5.10 ± 1.07 vs. 10.85 ± 0.93), flow rate (5.10 ± 1.07 vs. 10.85 ± 0.93) and total antioxidant capacity (87.63 ± 7.56 vs. 1110.33 ± 35.20) in thalassemia patients compared to controls, with all differences being statistically significant (p<0.05). The findings suggest that beta-thalassemia major adversely affects salivary composition and flow, impairing oral health defenses and increasing vulnerability to microbial infections. These results underscore the importance of routine oral health evaluations and interventions in managing dental care for children with thalassemia, emphasizing the need for comprehensive strategies that encompass both dental treatment and parental education to mitigate associated risks.
Keywords
Thalassemia; Salivary Immunoglobulin A (sIgA); Oral Health; Iron Overload; Antioxidant capacity
Introduction
Global Thalassemia is a genetic haemoglobin disorder found globally, with the highest prevalence in South East Asia and Africa. In India, the average prevalence is 3.3%.According to World Health Organization (WHO) data, approximately 7% of the global population carries a haemoglobin disorder and each year about 300,000-500,000 children are born with severe homozygous forms of these conditions [1,2].
Thalassemia major usually occurs within the first two years of life and is characterized by severe anemia, which requires regular Red Blood Cell (RBC) transfusions. The Thalassemia International Federation estimates that around 200,000 patients with thalassemia major are currently alive and receiving consistent treatment worldwide. Patients with beta-thalassemia major depend on routine blood transfusions for survival [3]. These transfusions cause the destruction of red blood cells, which increases ferritin levels in the body and leads to iron deposition in various tissues, including the salivary glands [4].
Human saliva contains a complex peroxidase system, primarily composed of various forms of lactoperoxidase secreted by the salivary glands and myeloperoxidase from polymorphonuclear neutrophils. Both cells and biological fluids are equipped with protective antioxidant mechanisms to prevent the production of free radicals and repair oxidative damage. These antioxidant systems include enzymes such as superoxide dismutase, catalase and glutathione peroxidase; macromolecules like albumin, ceruloplasmin and ferritin; and small molecules such as ascorbic acid, alpha-tocopherol, beta-carotene, reduced glutathione, uric acid and bilirubin. The combined effects of endogenous antioxidants and those obtained from food determine the total antioxidant capacity of the system [5].
Secretory Immunoglobulin A (sIgA) is the primary immunoglobulin found in external secretions that cover mucosal surfaces, such as those in the respiratory, intestinal and reproductive tracts. It plays an important role in the immune system’s “first line of defense” against microbial threats by preventing microbial adherence, neutralizing enzymes, toxins and viruses. It can also work in conjunction with other factors, like lysozyme and lactoferrin, to enhance oral immunity [6].
Ferritin accumulation in the salivary glands induces the damage of salivary glands and causes drying mouth, consequently affecting the components of saliva which function in caries protection. Damage to the salivary glands results in a reduced salivary flow rate and dryness of the mouth [7]. This decrease in salivary flow affects inorganic components like bicarbonates and phosphates, which are essential for neutralizing the oral cavity's pH. Given the limited research on salivary IgA levels in thalassemia patients and considering saliva's potential as a valuable non-invasive diagnostic tool, this study aims to compare salivary IgA levels, pH, buffer capacity, flow rate and total antioxidant capacity between children with β-thalassemia and healthy controls [4].
Materials and Methods
This study was carried out in the Department of Pedodontics and Preventive Dentistry in collaboration with the Sankalpa Voluntary Organization for thalassemic children, Khammam, Telangana.
Inclusion criteria:
• Children aged between 5 years and 12 years.
• Previously diagnosed with beta thalassemia major.
• Age and sex matched with healthy controls.
• Free from systemic or local diseases affecting salivary secretions (e.g., submandibular duct canaliculi, asthma, diabetes mellitus).
Exclusion criteria:
• Currently undergoing dental treatment.
• Suffering from conditions known to affect salivary parameters, such as diabetes or congenital heart defects (CHDs).
• Physically or medically compromised.
• On medications that might influence saliva, such as antipyretics, bronchodilators, or multi-vitamin syrups, which can lead to bacterial infections and dental caries if not properly rinsed.
• Using fluoride toothpaste or practicing improved oral hygiene measures aimed at preventing caries.
The study consisted of 40 children in study group and 40 children in control group, both in the age group of 5 years-12 years. Study group was taken from previously diagnosed patients suffering from beta thalassemia major and control group consisted of normal unaffected child.
For each patient, a hematological and odontostomatologic questionnaire was completed to have their age, sex, mean pretransfusion Hb level in the last three months, last ferritin level, fasting blood sugar, calcium and phosphate levels and thyroid function tests recorded.
Saliva samples
Unstimulated saliva samples were collected according to Dawes method. To ensure standardisation of samples and minimise the effect of diurnal variation, the saliva collection was carried out at the same time of the day between 9 Am to 11 Am on a routine basis. Prior information was provided to the subjects to refrain from eating and drinking at least 60 min before the collection. Salivary IgA is measured by Human IgA (Immunoglobulin A) Enzyme-Linked Immunosorbent Assay (ELISA) Kit (Cat: 3011255). Salivary samples were collected in a preweighed graduated cylinder and the flow rate was calculated immediately. Salivary pH and buffer capacity were measured by saliva check buffer kit. Total antioxidant capacity is measured by the phosphomolybdenum method and calorimeter.
Results
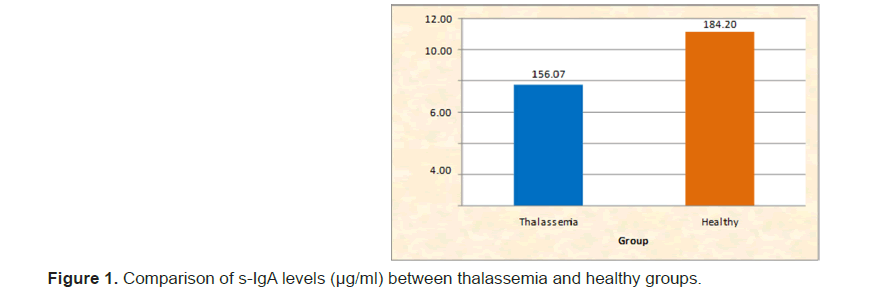
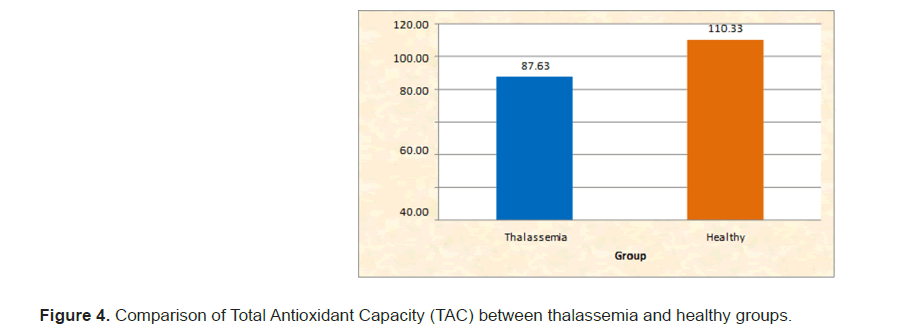
The salivary IgA levels, pH, buffer capacity, flow rate and total antioxidant capacity in children with beta thalassemia major compared to healthy controls were analyzed using IBM SPSS Statistics 20.0. Descriptive statistics and the Mann–Whitney test were applied. The mean salivary IgA levels differed significantly between the two groups. The mean ± SD for the thalassemia group was 156.07 ± 30.27, while for the healthy children, it was 184.20 ± 33.25. (Table 1 and Figure 1). The Significant difference in pH levels between the groups. The mean ± SD for thalassemia was 6.42 ± 0.17, compared to 7.00 ± 0.13 for healthy children. (Table 2 and Figure 2). The significant difference in buffer capacity. The mean ± SD for the thalassemia group was 5.10 ± 1.07, while it was 10.85 ± 0.93 for the healthy controls. (Table 3 and Figure 3). The significant difference in saliva flow rate. The mean ± SD for the thalassemia group was 5.10 ± 1.07 and for the healthy children, it was 10.85 ± 0.93. (Table 4 and Figure 4).
| Variable | Group | Min | Max | Mean | SD | Mann Whitney | |
|---|---|---|---|---|---|---|---|
| U value | p value | ||||||
| s-IgA levels (µg/ml) | Thalassemia | 60 | 95 | 156.07 | 30.27 | <0.001 | |
| Healthy | 105 | 150 | 184.2 | 33.25 | 200.5 | S | |
Table 1: Systematic search results.
| Variable | Group | Min | Max | Mean | SD | Mann Whitney | |
|---|---|---|---|---|---|---|---|
| U value | p value | ||||||
| pH | Thalassemia | 6.2 | 6.6 | 6.42 | 0.17 | 20 | <0.001 |
| Healthy | 5.5 | 8.5 | 7 | 0.13 | S | ||
Table 2: Comparison of pH between thalassemia and healthy groups
| Variable | Group | Min | Max | Mean | SD | Mann Whitney | |
|---|---|---|---|---|---|---|---|
| U value | p value |
||||||
| BC | Thalassemia | 3.6 | 6.4 | 5.1 | 1.07 | 0 | <0.001 |
| Healthy | 9 | 12 | 10.85 | 0.93 | S | ||
Table 3: Comparison of Buffering Capacity (BC) between thalassemia and healthy groups.
| Variable | Group | Min | Max | Mean | SD | Mann Whitney | |
|---|---|---|---|---|---|---|---|
| U value | p value | ||||||
| TAC | Thalassemia | 76 | 99.8 | 87.63 | 7.56 | 0 | <0.001 |
| Healthy | 69.2 | 151.7 | 110.33 | 35.2 | S | ||
Table 4: Comparison of Total Antioxidant Capacity (TAC) between thalassemia and healthy groups.
Discussion
The Sialometry and sialochemistry are valuable tools for diagnosing systemic illnesses, monitoring general health and assessing disease risk, highlighting the connection between oral and systemic health. Saliva in normal quantity and composition are important for the host immune system's defense against oral diseases [1,8].
Studies in the recent years indicate that Salivary Secretory IgA (s-IgA) is vital for local immunity. It binds to microbial surface molecules, such as those from caries-causing bacteria, preventing their adhesion to tooth surfaces. Additionally, s-IgA reduces bacterial surface hydrophobicity and neutralizes harmful components like Streptococcus mutans toxins and Glucosyltransferase (GTF), rendering them inactive [9].
One theory suggests that s-IgA prevents bacterial adhesion to tooth surfaces, neutralizes certain bacterial toxins and enzymes and works with other salivary proteins like lactoferrin or lysozyme to prevent caries. However, another theory posits that high levels of s-IgA may not correlate with reduced caries susceptibility, as individuals with higher s-IgA levels sometimes have more dental caries compared to those with lower levels, indicating a potential concentration-effect relationship [6].
Currently, methods commonly used for measuring s-IgA concentration in saliva include immunoturbidimetry, Radioimmunoassay (RIA) and Enzyme-Linked Immunosorbent Assay (ELISA). Among these, ELISA is preferred in clinical settings due to its specificity, sensitivity, ease of use, reagent stability and environmental safety. In this study, β-thalassemia major patients were found to have lower levels of Secretory Immunoglobulin A (sIgA). These antibodies are important for regulating oral microbiota by preventing bacterial adherence to the oral mucosa and teeth. The decrease in sIgA levels impacts the immune system's ability to protect the oral cavity from microorganisms [10,11].
The findings of this study align with Yousif SS et al., who observed lower mean values of secretory IgA in thalassemia patients compared to a control group, although these differences were statistically insignificant (p>0.05) [12]. Their study, however, was focused on dental caries. Conversely, our results contradict those of Ranadheer E and Nayak UA, et al., who found that increased levels of s-IgA were associated with a higher incidence of dental caries, suggesting a protective role of s-IgA against caries and Streptococcus mutans, which is active in cariogenic environments [12,13].
Regarding salivary pH, our findings are consistent with Dewi SR et al., with a mean of 6.34 ± 0.34 [4]. However, our results differ from those of Al Jobouri, who found the salivary pH in β-thalassemia major patients to be neutral at 7.17. The discrepancy may be due to different saliva collection methods: Al Jobouri used stimulated saliva, whereas unstimulated saliva was used in the present study [14].
Several factors could affect the salivary pH in these patients. A reduced salivary flow rate, which can result from routine blood transfusions, lowers the concentration of buffering components like bicarbonate and phosphate in the saliva. Additionally, frequent blood transfusions, typically performed at least once a month, can lead to iron overload in organs, including the salivary glands (hemosiderosis) [15]. This condition may cause inflammation and blockage in the salivary glands, further reducing salivary flow. Another theory is that iron accumulation in the salivary glands diminishes saliva production and secretion in β-thalassemia major patients [4].
The results of this study are consistent with those of Diwan and Mohammad, who found that β-thalassemia major impacts salivary flow rate [16]. Lactoferrin helps protect against caries by binding to Fe3+ ions necessary for microbial growth. However, due to reduced salivary flow in β-thalassemia patients, lactoferrin levels decrease, disrupting this ion-binding mechanism. Consequently, an excess of free Fe3+ ions can promote microbial growth in the oral cavity, leading to tooth decay. Lactoferrin also serves as a important host defense protein in the immune system.
Our study found that total antioxidant levels were lower in children with thalassemia compared to healthy controls, aligning with Salih et al., Thalassemia is characterized by defective synthesis of either the alpha or beta globin chains, which impairs iron binding and reduces oxygen transport to tissues [17]. As a compensatory response, ineffective erythropoiesis occurs in the bone marrow, leading to an overproduction of erythroblasts and excessive iron in the serum.
In normal cellular respiration, hydrogen peroxide is produced as a by-product of mitochondrial oxidative processes, which then generates hydroxyl radicals (OH−) through Fenton’s reaction:
Fe2++H2O2→Fe3++HO-+HO
These free radicals can deplete the body's antioxidant Reserves, Increasing Oxidative Stress (ROS) and leading to vascular tissue damage [5]. Given these findings, it is important to gather comprehensive information about thalassemia in children before proceeding with dental care. Our study highlights various oral health issues faced by thalassemic children. Therefore, preventive and therapeutic strategies should focus not only on dental treatment but also on raising awareness and educating parents about dental diseases and their management.
Conclusion
In the current study, children with β-thalassemia exhibited lower levels of salivary IgA, pH, buffer capacity, flow rate and total antioxidant capacity compared to healthy children.
Patients with beta-thalassemia major require regular blood transfusions to survive. These transfusions lead to the breakdown of red blood cells, which results in increased ferritin levels in the body. Excess ferritin can accumulate in various tissues, including the salivary glands, thus damaging them, reducing the salivary flow and causing dry mouth. The decrease in saliva flow impacts the inorganic components, such as bicarbonate and phosphate, which are essential for neutralizing the pH in the oral cavity. Additionally, Secretory Immunoglobulin A (sIgA) levels are found to be low in these patients. sIgA plays an important role in managing oral microbiota by inhibiting bacterial adhesion to the oral mucosa and teeth. A reduction in sIgA compromises the immune defense of the oral cavity, making it more vulnerable to microbial infections.
Ethical Committe
After ethical approval was granted by the ethical review committee and Institutional Review Board (MDC_ KT_20205103004D) and informing the parents about the study's objectives and procedures, only those children who provided written consent were included in the evaluation.
References
- Rani ST, Reddy ER, Kiranmai M, Mudusu SP, Srikanth S, et al. Comparative evaluation of BMI, dental age, salivary alkaline phosphatase levels and oral health status in children with β thalassemia major. Int J Clin Pediatr Dent. 2019;12:303-5.
[Crossref] [Google Scholar] [PubMed]
- Diwan JM, Mohammad ZJ. Study of salivary IgA concentrations, salivary flow rate in patients with β–thalassemia major in missan Governorate. J Bagh Coll Dent. 2015;27:55-7.
- Galanello R, Origa R. Beta-thalassemia. Orphanet J Rare Dis. 2010;5:1-5.
[Crossref] [Google Scholar] [PubMed]
- Dewi SR, Septhimoranie S, Muchzal S. Correlation of saliva characteristics and caries in beta-thalassemia major patients. J Dent Res. 2020;6:100-5.
- Rao GV, Preethi V, Daneswari V, Reddy V, Sivakalyan V, et al. A comparative study of salivary flow rate, pH, buffer capacity, total antioxidant capacity and ferritin levels in children with beta thalassemia major and healthy children. Int J Clin Pediatr Dent. 2021;14:342-3.
[Crossref] [Google Scholar] [PubMed]
- Wu Z, Gong Y, Wang C, Lin J, Zhao J. Association between salivary s-IgA concentration and dental caries: an updated meta-analysis. Bioscience Reports. 2020;40:23-5.
[Crossref] [Google Scholar] [PubMed]
- Lynge Pedersen AM, Belstrom D. The role of natural salivary defences in maintaining a healthy oral microbiota. J Dent. 2019; 8:3-12.
[Crossref] [Google Scholar] [PubMed]
- Bellagambi FG, Lomonaco T, Salvo P, Vivaldi F, Hangouët M, et al. Saliva sampling: Methods and devices. An overview. Int J Oral Health Dent. 2017;3:149-53.
- Lamont RJ, Hajishengallis GN, Koo HM, Jenkinson HF. Oral Microbiology and Immunology.2nd ed. New Jersey:John Wiley and Sons;2020:214-7.
- Marengo-Rowe AJ. The thalassemias and related disorders. Proc Bayl Univ Med Cent. 2007;20:27-31.
[Crossref] [Google Scholar] [PubMed]
- Arora R, Malik S, Arora V, Malik R. Comparison of dental caries prevalence in Β Thalassemia major patients with their normal counterparts in Udaipur. Am Int J Res Sci. 2014;5:6-9.
- Yousif SS, Salih BA. Evaluation of salivary immunoglobulin a and iron ion in relation to dental caries among children with beta thalassemia major. An. Romanian Soc Cell Biol. 2021;25:314-8.
- Ranadheer E, Nayak UA, Reddy NV, Rao VA. The relationship between salivary IgA levels and dental caries in children. J Indian Soc Pedod Prev Dent. 2011;29:106-7.
- Al-Jobouri HS, Al-Casey M. Selected salivary constituents among 16-18 years patients with β thalassemia major in relation to oral diseases. J Bagh Coll Dent. 2011;23:124-7.
- Kumar B, Kashyap N, Avinash A, Chevvuri R, Sagar MK, et al. The composition, function and role of saliva in maintaining oral health: A review. Int J Contemp Dent Med Rev. 2017;33:1-7.
- Fine DH. Lactoferrin: A roadmap to the borderland between caries and periodontal disease. J Dent Res. 2015;94:768-76.
[Crossref] [Google Scholar] [PubMed]
- Salih KM, AL-Mosawy WF, Faraj YF, Jouda J. Investigation of antioxidant status in Iraqi patients with beta thalassemia major. J Glob Pharma Technol. 2017;7:109-13.






 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.