Comparing between Hydroxyurea and Non-Hydroxyurea Anti-inflammatory Cytokines in Sickle Cell Anemia Saudi Patients
2 Department of Biology, Jeddah University, Jeddah, Saudi Arabia
3 Department of Hematology, King Abdulaziz University, Jeddah, Saudi Arabia
Published: 30-Sep-2021
Citation: Tarbiah NI, et al. Comparing between Hydroxyurea and Non-Hydroxyurea Anti-inflammatory Cytokines in Sickle Cell Anemia Saudi Patients. Ann Med Health Sci Res. 2021;11:39-44.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Sickle Cell Anemia SCA is a hemolytic deficiency that is typified by strangely formed sickles red blood cells that are expelled from circulation at expanded rates, leading to anemia. It is caused by inheritance of hemoglobin Hb that has mutated, which compared to adult normal Hb, contains a single amino acid substitution, where at the sixth residue valine substitutes glutamic acid in the Hb tetramer in both of the two B chains. For SCA patients, chronic inflammatory conditions are linked to high levels of inflammation markers, including cytokines and adhesion molecules. Cytokines are a group of small proteins that are essential in cell signaling. The immuno regulatory molecules known as anti-inflammatory cytokines control the response of pro inflammatory and have gained attention recently as anti-inflammatory mediator in SCA patients. The most interesting anti-inflammatory cytokines are IL10, IL 4, IL 27, and IL 37. This a case-control study aimed to measure the concentrations of the antiinflammatory cytokines in 3 groups, SCA patients without the use of any medication, SCA patients under Hydroxy Urea therapy SCAHU, and healthy people male and female in the general Saudi population, using a total of 78 chosen subjects. Using ELISA kits, cytokine levels were measured in the plasma of the patients. The results of the study indicated that anti inflammatory cytokines were elevated in SCA and SCAHU patients when compared to the control P equal to 0.001, and there were no significant differences between SCA and SCAHU patients. The anti inflammatory cytokines, including IL 27 and IL 37, that were detected for the first time in SCA patients in KSA in this research, have a role in keeping patients in a steadystate and reducing inflammation, therefore more research to understand the roles of these cytokines in SCA pathology of is essential to allow the development of effective therapies to treat this disease.
Keywords
Anti-inflammatory cytokines; Hydroxyurea; Inflammation; Sickle cell disease
Introduction
Sickle Cell Anemia (SCA) results from Hemoglobin S (HbS) inheritance, which is mutated hemoglobin that has a single amino acid substitution compared with normal adult hemoglobin, HbA, as at the sixth residue of the two B chains valine replaces glutamic acid in the Hb tetramer. On the HbS protein surface, there is a loss of a negative charge at a crucial position which, upon deoxygenation, allows it to polymerize to form long and rigid rods. The shape change results in sickling, and other harmful sequelae and cause the multiple clinical signs associated with SCA. [1] Sickled cells are not only less pliable than healthy red blood cells, but they are also stickier. This leads sickle cells to adhere to each other and clog narrow blood vessels, limiting blood flow and preventing adequate oxygen from being delivered to the tissues that need it. Red blood cells’ sickle phenomena are influenced by many environmental and psychosocial agents, including hypoxemia and dehydration. [2] Complications in patients with SCA are categorized depending on the predominant pathophysiology, into vaso-occlusion sub-phenotypes and haemolytic endothelial dysfunction. In patients with SCA, chronic inflammatory states can be linked to increased inflammation expression levels including adhesion molecules and cytokines. This is due to complex interactions between endothelial cells, erythrocytes, leukocytes and platelets. [3] Increased levels of pro-inflammatory cytokines combined with increased acute-phase proteins, including C-Reactive Protein (CRP), and reduced levels of anti-inflammatory cytokines help to characterize the inflammatory phenotype in the stable status during Vaso-Occlusive Crises (VOC) for patients. During this genetic disease, the released cytokines then stimulate vascular endothelium activation, along with activation of the blood components, sickle cells, platelets, reticulocytes, leukocytes, and these adhere to the vascular wall using different mechanisms, including the induction of expression of fibronectin expression, Vascular Cell Adhesion Molecule (VCAM) production of chemokines, and there is also deregulation of apoptosis in the endothelial cells, which generates a vicious cycle that results in VOC. [4] Cytokines are produced by different types of cells and often display overlapping activities, regulating differentiation or proliferation, depending on the type and developmental state of the involved target cells. [5] There is evidence for an altered balance between anti-inflammatory and pro-inflammatory cytokines in SCD patients during VOC, there are reduced levels of anti-inflammatory cytokines and increased pro-inflammatory cytokines levels when compared to healthy individuals. [6]
Pro-inflammatory cytokine response is controlled by anti-inflammatory cytokines, which are a group of specific immuno-regulatory molecules. The human immune response is regulated by cytokines which work in tandem with soluble cytokine receptors and specific cytokine inhibitors. There is increasing recognition of the importance of their pathologic role in systemic inflammatory and physiologic role during inflammation. [7] Hydroxy Urea (HU) therapy that has proved to be effective for SCA patients with frequent symptoms. Also, it is known to raise HbF levels, improve mean corpuscular volume, concentrations of hemoglobin, and minimize reticulocytes numbers. Another positive result of therapy is that it not only minimizes adhesion molecule expression, it also reduces receptor protein numbers on endothelial cells. Overall, this suggests HU reduces vascular adhesion, which diminishes the number of VOCs. [8]
Aim of the study
The aim a case-control study of this research was to measure the concentrations of the anti-inflammatory cytokines (IL-10, IL-4, IL-27 and IL-37) in patients with sickle cell anemia (male and female) in the general Saudi population, and compare them to different groups of patients as follows: Sickle Cell Anaemia patients without the use of any medication (SCA), patients under Hydroxy Urea therapy (SCAHU), and healthy people, to try and understand their role in the development of the disease and understand how HU therapy effect on these cytokines.
Materials and Methods
Human subjects
All protocols and consent forms were approved in Jeddah, by the ethical committees on research from biomedical ethics, King Abdulaziz hospital university. The research included 78 males and females, 22 of them were control individuals and the rest were divided into two groups: steady-state SCA patients and SCA patients under HU therapy. They were diagnosed and followed up at the hematology and hemotherapy center of King Abdulaziz university hospital. Each of them was issued a written informed consent of their participation in the study. Blood samples of participants were taken by the medical hematology and hemotherapy center of King Abdulaziz university hospital, and blood samples for the control group were collected from volunteers in King Fahad Medical Research Center. 5 mL of venous blood was collected into EDTA tubes from each patient and a Complete Blood Count (CBC) was taken for all participants. The blood samples were centrifuged for 15 minutes and then stored, the plasma separated into eppendorf and stored at -80°C until analysed. The plasma levels of the IL-10 IL-4, IL-27, and IL-37 were quantified by Enzyme-Linked Immunosorbent Assay (ELISA) using commercial kits (elabscience, USA) following the manufacturer’s instructions.
Detection of anti-inflammatory cytokines
The following ELISA kits were used in this study; elabscience human IL-37 ELISA kit Cat. No.: E-EL-H2571, elabscience human IL-27 ELISA kit Cat. No.: E-EL-H2338. Elabscience human IL-4 ELISA kit Cat. No.: E-EL-H0101 elabscience human IL-10 ELISA kit Cat. No.: E-EL-H0103.
Statistical analysis
The Statistical Package for Social Sciences (SPSS version 22) was used. Data were presented as mean ± Standard Error of the Mean (SEM). For the comparison of the data, kruskal-wallis test was used to compare between more than two groups, while for comparisons between two groups, the Mann-Whitney U test was used, and the P-values ≤ 0.05 were considered significant.
Results
Patients’ demographical characteristics
The participants’ demographic and hematological characteristics of the controls and patients [Table 1] show there were statistically significant differences between controls and the SCA group where are RBC, Hematocrit (HCT), and Hemoglobin (Hb) decrease in SCA patients, while WBC, platelets, lymphocytes, neutrophils, monocytes, lymphocytes, basophils, and eosinophils increase as the p-values are less than 0.05. Also, significant differences between controls and the SCAHU group where are RBC, Hematocrit (HCT), and Hemoglobin (Hb) decrease in the SCAHU group, while an increase of WBC, Mean Corpuscular Volume (MCV), Mean Corpuscular Hemoglobin (MCH), neutrophils, lymphocytes, monocytes, eosinophils, and basophils as the p-values are less than 0.05. In addition, there were significant differences between SCA and SCAHU groups where RBC, Hb, HCT are, and platelets decrease in the SCAHU group as the p-values are less than 0.05.
| Table 1: The participants’ demographic and hematological characteristics of the controls and patients. | |||
|---|---|---|---|
| Parameters | Control (n=22) A |
SCAHU (n=30) B |
SCA (n=26) C |
| Hemoglobin (Hb) (g/dl) | 12.8 ± 1.2 | 7.5 ± 0.7 | 8.8 ± 1.2 |
| Hematocrit (HCT) (%) | 38.2 ± 3.2 | 21.9 ± 1.8 | 26.7 ± 4.6 |
| Mean Corpuscular Hemoglobin (MCH) (pg) | 27.9 ± 2.8 | 30.5 ± 2.1 | 27.9 ± 4.5 |
| Mean Corpuscular Volume (MCV) (fl) | 83.2 ± 6.8 | 93.3 ± 9.7 | 83.3 ± 9.7 |
| Mean Corpuscular Hemoglobin Concentration (MCHC) (g/dl) | 33.4 ± 1.0 | 34.1 ± 1.2 | 33.4 ± 2.2 |
| Platelets (10^3/µL) | 287.7 ± 45.6 | 342.6 ± 144.6 | 515.8 ± 142.5 |
| Neutrophils (10^3/µL) | 2.5 ± 0.7 | 5.9 ± 2.1 | 5.53 ± 2.5 |
| Lymphocytes (10^3/µL) | 2.2 ± 0.5 | 4.01 ± 0.9 | 4.66 ± 2.6 |
| Monocytes (10^3/µL) | 0.5 ± 0.1 | 1.1 ± 0.4 | 0.99 ± 0.46 |
| Eosinophil (10^3/µL) | 0.17 ± 0.12 | 0.4 ± 0.4 | 0.38 ± 0.27 |
| Basophil (10^3/µL) | 0.04 ± 0.02 | 0.1 ± 0.08 | 0.085 ± 0.04 |
| The results are expressed as mean ± SD; WBC: White Blood Cells; RBC: Red Blood Cells | |||
Anti- inflammatory cytokines detection
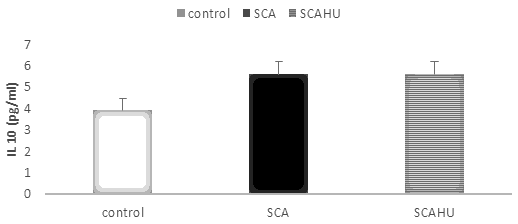
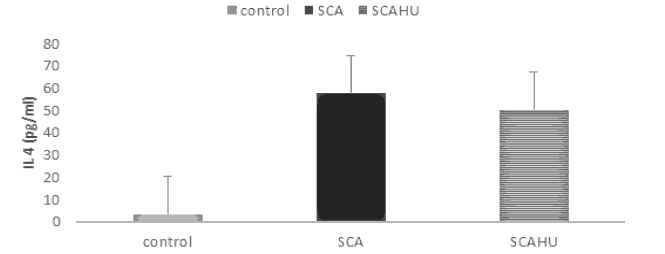
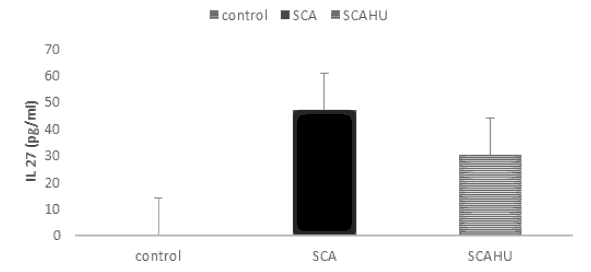
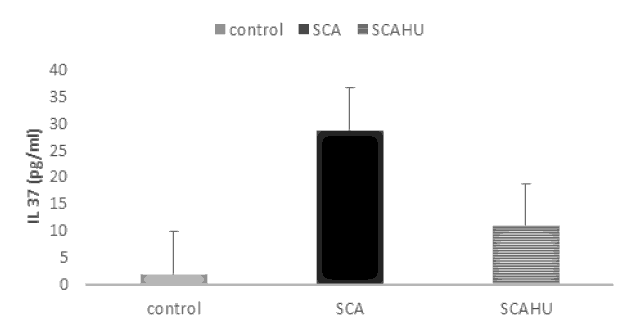
Our results showed significant differences between SCA group and the control group for IL-10 (p-value=0.003), IL-4 (p-value=0.001), IL-27 (p-value=0.001) and IL-37 (p-value=0.001). Also, the data showed that plasma IL-10 [Figure 1], IL-4 [Figure 2], IL-27 [Figure 3] and IL-37 [Figure 4] levels were significantly higher in the SCAHU group when compared to the control group (p-value=0.008, 0.001, 0.001, 0.001 respectively). There were no significant differences between the SCA and SCAHU were detected for all previous cytokines.
Figure 1 showed that IL-10 concentration was significantly higher in SCA and SCAHU patients than control individuals (p-value=0.003, p-value=0.008 respectively). No difference between patients' groups.
Figure 2 showed that IL-4 concentration was highest in SCA patients compared to SCAHU and control groups. Significant difference detected between SCAHU and control (P=0.001) and between SCA with control individuals (P=0.001) while no differences detected between SCA and SCAHU patients.
Figure 3 showed that IL-27 concentration was highest in SCA patients compared to SCAHU and control groups. Significant difference detected between SCAHU and control (P=0.001) and between SCA with control individuals (P=0.001) while no differences detected between SCA and SCAHU patients.
Figure 4 showed that IL-37 concentration was highest in SCA patients compared to SCAHU and control groups. Significant difference detected between SCAHU and control (P=0.00) and between SCA with control individuals (P=0.001). While no differences detected between SCA and SCAHU patients.
Discussion
Levels of IL-10 plasma, which various cell types produce, such as dendritic cells, lymphocytes, macrophages, and other leukocytes, and mainly work to inhibit many pro-inflammatory chemokines and cytokines from being produced, [9] were higher in SCA and SCAHU patients than in the control individuals, but there were no differences between SCA and SCAHU. Reports about the roles of the anti-inflammatory cytokines IL-4 and IL-10 in SCA patients have conflicting results. Some studies have previously shown that IL-4 and IL-10 were higher in SCAHU than SCA patients, whereas others showed no effect of HU.; the results for VOC patients also show conflicts; the study by Sarray et al. reported an association between chronic inflammation and VOC onset, and as IL-10 has the capacity to act as an anti-inflammatory, they found levels were higher in control individuals and in SCA steady-state patients than in VOC patients. These results support the idea that reduced availability of IL-10 causes increased inflammation during painful VOC episodes. This confirms its role as an anti-inflammatory mediator, and this agrees with our findings. [6] Studies have shown that HU decreased levels of the pro-inflammatory cytokine TNF-a, and increased levels of IL-10 in the plasma of SCD patients; this indicates HU may have a role in IL-10 regulation. However, reports of it’s the effects of HU on other key cytokines contain some inconsistencies. [9] Although our results did not indicate a significant effect of HU on IL-10, the study by Lanaro showed that production of IL-10 mRNA was-significantly increased in HU patient’s neutrophils, and there was a significant positive correlation between levels of HbF and IL-10 gene expression in neutrophils was found in SCAHU and SCA patients, which indicates there may be a direct role for HbF in increasing levels of the anti-inflammatory IL-10. [9] These results confirm the role of IL-10 as anti-inflammatory mediator in patients with SCA.
Another study conducted by Pitanga et al. demonstrated that there were high levels of IL-10 found in patients undergoing HU therapy, but how this occurred was not described. This result conflicts with our findings, which indicated there was no effect of HU, but this is somewhat consistent with previous studies and may require a larger group of patients for the differences to be detected. [10] The anti-inflammatory cytokine IL-4 is involved in immune system regulation at multiple levels. It promotes differentiation of TH2 cells and results in the differentiation inhibition for TH1 cells. IL-4 is also a survival and growth factor that works to help protect lymphocytes from undergoing apoptosis. IL-4 has an important role to play in allergic disease pathogenesis, especially for atopic asthma, and it is also plays an essential role during the immune response to parasitic infections. [11]
In this study, IL-4 plasma levels in SCA and SCAHU patients were significantly increased when compared to the controls, and slightly higher in SCA than in SCAHU patients, but this did not reach statistically significant levels. These results are somewhat consistent with previous studies and suggest the study may require a larger group of patients to see the results fully. Most previous studies have shown that for IL-4, there was no significant difference between controls and SCA patients; the study by Musa et al. which measured IL-4 levels in the serum of SCA steady-state, VOC, and control individuals showed that IL-4 was elevated in VOC patients, and there is no difference between SCA and control individuals. [12] This conflicts with our result and may be due to differences in plasma or serum cytokines measured. Another study by Pathare et al. showed that IL-4 was increased more in SCA patients than in control individuals (which correlate with our result); however, between SCA and VOC patients, there was no difference. [13] There is insufficient evidence in the literature about the effect of HU therapy on IL-4 levels to compare the results of this study to.
IL-27 and IL-37 have recently caught the attention of both scientists and researchers as anti-inflammatory biomarkers in SCA patients as IL-37 and IL-27 expression levels are low in healthy individuals. [14] IL-37 can be detected in many human tissues, such as liver, lung, thymus, placenta, lymph nodes, bone marrow, and others, and it is expressed by various immune and non-immune cells, including monocytes, macrophages, dendritic cells, and epithelial. [15,16] While after stimulation, IL-27 is produced by macrophages and dendritic cells. [17] In inflammatory diseases including uveitis, arthritis rheumatoid, and encephalomyelitis, IL-37 has been suggested to have a potential inhibitory role. [16]
In cultures pre-stimulated with IL-27 or IL-37, these anti-inflammatory cytokines were associated with inflammatory response inhibition that was induced by heme. Based on these findings, the use of IL-27 or IL-37 in diseases where there is a sudden heme concentration increase, such as in hemolytic anemia, may provide a protective effect and could ameliorate the innate immune response mediated clinical complications. This treatment may also have a beneficial role for chronic end organ morbidities and VOC. [18,19] The first study that showed increased concentrations of IL-27 and IL-37 in SCA patients was by Alagbe et al. who found that the levels of these cytokines appeared significantly higher in the SCA group when compared to the controls, but they did not find a significant difference between the SCA and SCAHU patients. [3] This result was highly comparable with our results, were IL-27 and IL-37 plasma levels were elevated in SCA and SCAHU patients when compared to the control individuals, but there were no significant differences between SCA and SCAHU patients.
The concentration of IL-37 and IL-27 tends to be higher in the SCA patients than in SCAHU patients; this may indicate that HU therapy acts as an anti-inflammatory agent and has no strong effect on them. These results agree with previous studies that showed IL-27 and IL-37 were increased in patients with different chronic inflammatory diseases, including rheumatoid arthritis, ankylosing spondylitis, atherosclerosis, and colitis. [20,21]
Finally, our findings combined with previous studies suggest that the heme induced chronic inflammatory state cause’s positive feedback that induces the secretion of pro-inflammatory cytokines and also negative feedback that induces the secretion of IL-27 and IL-37. [19] In the literature there are not sufficient studies of IL-27 andIL-37 so further research is required.
Conclusion
In conclusion, the analysis of the research results showed that anti-inflammatory cytokines were found in high concentrations for SCA patients and in SCA patients under HU therapy when compared to controls individuals. HU use did not alter anti-inflammatory cytokine secretion significantly in sickle cell anemia patients. Studies have shown that cytokines levels in SCA have been implicated in disease severity and the changes to the production of chemokines and cytokines can contribute to inflammatory and infectious disease pathogenesis. The anti-inflammatory cytokines detected in this research have a role in keep patients in a steady state. We recommend to more studies to help understand the roles of the different cytokines in SCA pathology, which will be essential for effective therapy development for this disease. Studies have suggested cytokines can be used as markers for disease severity, and also that genetic studies of the enzymes involved in disease severity, such as heme oxygenase, should be carried out.
Acknowledgments
The authors would like to express their thanks to King Abdulaziz University for their support of the scientific research. Also, they would like to express their very great appreciation for Dunya Nori for her valuable and constructive suggestions during the research work.
Conflict of Interest
No conflicts of interest
Financial Support
No
Ethics Statement
Biomedical Research Ethics Committee, Ministry of Higher Education, King Abdulaziz University, Faculty of Medicine.
References
- Balushi HWMA, Rees DC, Brewin JN, Hannemann A, Gibson JS. The effect of xanthine oxidase and hypoxanthine on the permeability of red cells from patients with sickle cell anemia. Physiol Rep. 2018;6:1-12.
- Ilesanmi OO. Pathological basis of symptoms and crises in sickle cell disorder: Implications for counselling and psychotherapy. Hematol Rep. 2010;2:e2-e2.
- Alagbe AE, Justo ASJ, Ruas LP, Tonasse WV, Santana RM, Batista THC, et al. Interleukin-27 and interleukin-37 are elevated in sickle cell anemia patients and inhibit in vitro secretion of interleukin-8 in neutrophils and monocytes. Cytokine. 2018;107:85-92.
- Toledo SLDO, Guedes JVM, Alpoim PN, Rios DRA, Pinheiro MDB. Sickle cell disease: Hemostatic and inflammatory changes, and their interrelation. Clinica Chimica Acta. 2019;493:129-137.
- Scheller J, Chalaris A, Arras DS, John SR. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813:878-888.
- Sarray S, Saleh LR, Saldanha FL, Habboubi HHA, Mahdi N, Almawi WY. Serum IL-6, IL-10, and TNFα levels in pediatric sickle cell disease patients during vasoocclusive crisis and steady state condition. Cytokine. 2015;72:43-47.
- Opal SM, DePalo VA. Anti-inflammatory cytokines. Chest. 2000;117:1162-1172.
- Gardner RV. Sickle cell anemia, a race specific disease. J Am Med Assoc. 1947;133:33-34.
- Lanaro C, Penteado CFF, Albuqueque DM, Saad STO, Conran N, Costa FF. Altered levels of cytokines and inflammatory mediators in plasma and leukocytes of sickle cell anemia patients and effects of hydroxyurea therapy. J Leukoc Biol. 2009;85:235-242.
- Pitanga TN, Boass WV, Cerqueira BAV, Seixas MO, Barbosa CG, Adorno EV, et al. Cytokine profiles in sickle cell anemia: Pathways to be unraveled. Adv Biosci Biotechnol. 2013;4:6-12.
- Taylor SC, Shacks SJ, Qu Z, Wiley P. Type 2 cytokine serum levels in healthy sickle cell disease patients. J Natl Med Assoc. 1997;89:753-757.
- Musa BOP, Onyemelukwe GC, Hambolu JO, Mamman AI, Isa AH. Pattern of serum cytokine expression and T-cell subsets in sickle cell disease patients in vaso-occlusive crisis. Clin Vaccine Immunol. 2010;17:602-608.
- 13. Pathare A, Kindi SA, Alnaqdy AA, Shahina D, Macauly HK, Dennison D. Cytokine profile of sickle cell disease in Oman. Am J Hematol. 2004;77:323-328.
- Jia H, Liu J, Han B, Reviews of interleukin-37: Functions, receptors, and roles in diseases. Biomed Res Int 2018:3058640-3058640.
- Gao W, Kumar S, Lotze MT, Hanning C, Robbins PD, Gambotto A. Innate immunity mediated by the cytokine IL-1 homologue 4 (IL-1H4/IL-1F7) induces IL-12-dependent adaptive and profound antitumor immunity. J Immunol Res. 2003;170:107-113.
- Boraschi D, Lucchesi D, Hainzl S, Leitner M, Maier E, Mangelberger D, et al. IL-37: A new anti-inflammatory cytokine of the IL-1 family. Eur Cytokine Netw. 2011;22:127-147.
- Cao Y, Doodes PD, Glant TT, Finnegan A. IL-27 induces a Th1 immune response and susceptibility to experimental arthritis. J Immunol. 2008;180:922-930.
- Ye L, Ji L, Wen Z, Zhou Y, Hu D, Li Y, et al. IL-37 inhibits the production of inflammatory cytokines in peripheral blood mononuclear cells of patients with systemic lupus erythematous: Its correlation with disease activity. J Transl Med. 2014;12:69.
- Banchereau J, Pascual V, Garra AO. From IL-2 to IL-37: The expanding spectrum of anti-inflammatory cytokines. Nat Immunol. 2012;13:925-931.
- Wu BW, Zeng QT, Meng K, Ji QW. The potential role of IL-37 in atherosclerosis. Pharmazie. 2013;68:857-860.
- Lai X, Wang H, Cao J, Li Y, Dai Y, Xiang Y, et al. Circulating IL-27 is elevated in rheumatoid arthritis patients. Molecules. 2016;21:1-10.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.