Efficacy of Immunomodulatory Biologic Treatments in Atopic Dermatitis Patients: Systematic Review and Meta-analysis
2 Medical Intern, Ibn Sina National College, Jeddah City, Saudi Arabia
3 Medical Intern, Dar AlUloom University, Riyadh City, Saudi Arabia
4 Medical Intern, King Saud University, Riyadh City, Saudi Arabia
Medical Intern, Almaarefa University, Riyadh City, Saudi Arabia
Citation: Alrumaidhi NB, et al. Efficacy of Immunomodulatory Biologic Treatments in Atopic Dermatitis Patients: A Systematic Review and Meta-analysis. Ann Med Health Sci Res. 2021;11: 1203-1207.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Background: Skin lesional from Atopic Dermatitis (AD) patients characterized by. elevated expression of the T2 cytokine axis takes place in ad, in which interleukin (IL)-4 and IL-13 play a major role. Aim: This work aims to determine the efficacy of immunomodulatory biologic treatments in atopic dermatitis patients. Materials and Methods: A systematic search was performed over different medical databases to identify Dermatology studies, which studied the outcome of the Biologic group versus the Placebo group of atopic dermatitis patients. Using the meta-analysis process, either with fixed or random-effects models, we conducted a meta-analysis on one of the following scores: eczema area and severity index (EASI), dermatology life quality index (DLQI) score and patient-oriented eczema measure (POEM) score as the main study outcomes. Results: Five studies were identified involving 1298 patients, with 834 patients in the Biologic group, and 464 patients in the placebo group. The meta-analysis process revealed that using the random-effects model, the meta-analysis process revealed a highly significant increase in mean EASI score in the Biologic group (p = 0.003). Using the random-effects model, the meta-analysis process revealed a non-significant difference in mean DLQI and POEM scores in the Biologic group (p < 0.05). Conclusion: To conclude, inflammatory disease, characterized by intense itching and eczema. Patients may need immunobiological therapies to achieve proper disease control.
Keywords
Immunomodulatory; Biologic; Atopic dermatitis
Introduction
Atopic dermatitis (AD) is a common inflammatory skin disorder, with an occurrence of 2–8% in the person population and up to 20% in infants in maximum countries around the world. Affected patients suffer from persistent or relapsing pores and skin lesions related to a spectrum of atopic comorbidities. Ad negatively affects the fine of lifestyles (QoL) of patients in fitness-related aspects such as bodily, psychosocial, and mental functioning. Skin lesional from AD patients characterized by elevated expression of the T2 cytokine axis takes place in ad, in which interleukin (IL)-4 and IL-13 play a major role. [1]
AD is a diagnosis based totally on clinical presentation. current studies detailing the underlying mechanisms of ad 18 holds hope that biomarkers may be available to confirm the diagnosis and possibly differentiate numerous ad phenotypes (e.g., pediatric ad, Asian-origin ad), but the ad is mostly diagnosed by signs and symptoms and exclusion. [2]
Pruritus is an early feature of the ad. The ad is commonly related to intense nocturnal pruritus, which can affect sleep and quality of existence (QoL). Sleep insufficiency can result in excessive daylight hour’s sleepiness, mood disturbance, and impaired cognition, which could then affect work or school productivity, accidents, and adverse fitness outcomes such as metabolic, endocrine, and immune dysregulation which include type 2 diabetes, hypertension, and infection. [3]
Systemic immunomodulatory agents used to treat ad consist of the older medications cyclosporine, methotrexate, azathioprine, and mycophenolate and the biologic dupilumab. Several biologic and small-molecule medicines are being studied in scientific trials. Understanding the relative effectiveness and safety of various treatments is challenging because most have not been as compared head to head. A systematic review of randomized clinical trials (RCTs) published in 2014 did not include those novel therapies or a quantitative synthesis. [4]
This work aims to determine the efficacy of immunomodulatory biologic treatments in atopic dermatitis patients.
Literature Review
Our review came following the (PRISMA) statement guidelines. [5]
Study eligibility
The included studies should be in English, a journal published article, and a human study describing atopic dermatitis patients. The excluded studies were non-English or animal studies or describing other types of immunomodulatory drugs (e.g. immunosuppressive drugs).
Study identification
Basic searching was done over the PubMed, Cochrane library, and Google scholar using the following keywords: Immunomodulatory, Biologic, Atopic dermatitis.
Data extraction and synthesis
RCTs, clinical trials, and comparative studies, which studied the outcome of the Biologic group versus Placebo group of atopic dermatitis patients, will be reviewed.
Outcome measures included the following scores: Eczema Area and Severity Index (EASI), Dermatology Life Quality Index (DLQI) score, and Patient-Oriented Eczema Measure (POEM) score as the main study outcomes).
Study selection
We found 250 records, 190 excluded based on title and abstract review; 60 articles are searched for eligibility by full-text review; 25 articles cannot be accessed; 12 studies were reviews and case reports; the desired drugs not used in 18 studies leaving 5 studies that met all inclusion criteria.
Statistical analysis
After the pooling of data, Odds ratios (OR), Standard Mean Differences (SMDs), Proportions (%), with 95% confidence intervals (CI) were calculated, using MedCalc statistical software (Belgium). After the Q test of heterogeneity, the I2- statistics (either the fixed-effects model or the random-effects model) were done within the meta-analysis process.
Results
The included studies were published between 2016 and 2020 [Table 1]. [6-10]
| N | Author | Type of biologic drug used |
Number of patients | Age (average years) |
Disease duration (average years) |
||
|---|---|---|---|---|---|---|---|
| Total | Biologic group | Placebo group | |||||
| 1 | Thaçi et al. [6] | Dupilumab | 124 | 63 | 61 | -- | 29.8 |
| 2 | Eric L. Simpson et al. [7] | Lebrikizumab | 209 | 156 | 53 | 35 | -- |
| 3 | Guttman-Yassky et al. [8] | Baricitinib | 124 | 75 | 49 | 36 | 22 |
| 4 | Silverberg et al. [9] | Nemolizumab | 226 | 169 | 57 | 40 | -- |
| 5 | E. L. Simpson et al. [10] | Baricitinib | 615 | 371 | 244 | 35 | 26 |
| #Studies arranged via publication year. | |||||||
Table 1: Patients and study characteristics.
Regarding patients’ characteristics, the total number of patients in all the included studies was 1298 patients, with 834 patients in the Biologic group, and 464 patients in the placebo group [Table 1].
The mean age of all patients was (36.5 years), and the average disease duration was (25.9) years [Table 1].
A meta-analysis study was done on 5 studies that described and compared the 2 different groups of patients; with an overall number of patients (N=1298) [Table 2]. [6-10]
| N | Author | Main outcomes | |||||
|---|---|---|---|---|---|---|---|
| EASI score | DLQI score | POEM score | |||||
| Biologic group | Placebo group | Biologic group | Placebo group | Biologic group | Placebo group | ||
| 1 | Thaçi et al. [6] | 25.6 | 7.2 | 4.3 | 11.4 | -- | -- |
| 2 | Eric L. Simpson et al. [7] | 58.5 | 53.1 | 34.3 | 33.6 | -- | -- |
| 3 | Guttman-Yassky et al. [8] | 60 | 40 | 55 | 38 | 52 | 35 |
| 4 | Silverberg et al. [9] | 59 | 44 | -- | -- | -- | -- |
| 5 | E. L. Simpson et al. [10] | 33 | 33 | 14 | 15 | 20 | 21 |
Table 2: Summary of outcome measures in all studies.
Each outcome was measured by:
Standard Mean Difference (SMD)
• For the EASI score.
• For the DLQI score.
• For POEM score.
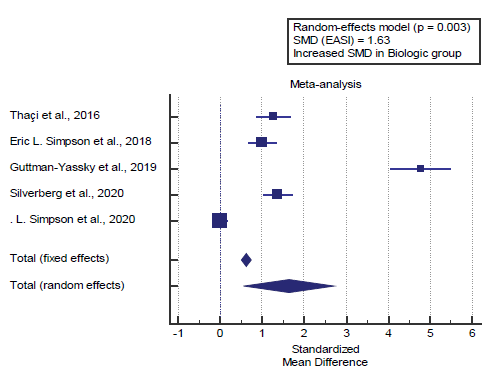
Concerning the main outcome measures, we found 5 studies reported the EASI score. I2 (inconsistency) was 98.2% with a highly significant Q test for heterogeneity (p<0.0001), so random-effects model was carried out; with overall SMD= 1.63 (95% CI=0.563 to 2.714).
Using the random-effects model, the meta-analysis process revealed a highly significant increase in mean EASI score in the Biologic group compared to the placebo group (p=0.003) [Figure 1].
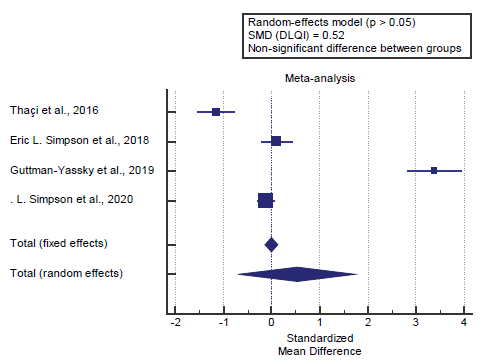
We found 4 studies reported the DLQI score. I2 (inconsistency) was 98.3% with a highly significant Q test for heterogeneity (p<0.0001), so random-effects model was carried out; with overall SMD= 0.52 (95% CI=-0.685 to 1.742).
Using the random-effects model, the meta- analysis process revealed a non-significant difference in mean DLQI score in the Biologic group compared to the placebo group (p>0.05) [Figure 2].
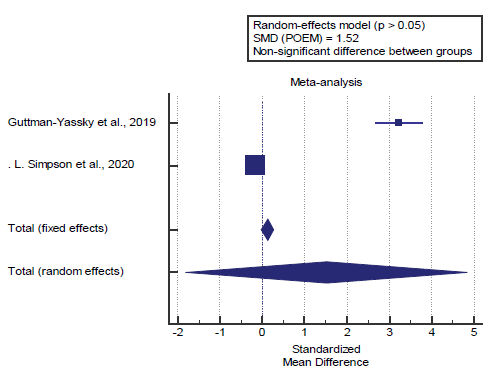
We found 2 studies reported the POEM score. I2 (inconsistency) was 99.3% with a highly significant Q test for heterogeneity (p<0.0001), so random-effects model was carried out; with overall SMD= 1.52 (95% CI=-1.799 to 4.847).
Using the random-effects model, the meta-analysis process revealed a non-significant difference in mean POEM score in the Biologic group compared to the placebo group (p>0.05) [Figure 3].
Discussion
This work aims to determine the efficacy of immunomodulatory biologic treatments in atopic dermatitis patients.
Regarding patients’ characteristics, the total number of patients in all the included studies was 1298 patients, with 834 patients in the Biologic group, and 464 patients in the placebo group. The mean age of all patients was (36.5 years), and the average disease duration was (25.9) years.
A meta-analysis study was done on 5 studies that described and compared the 2 different groups of patients; with an overall number of patients (N=1298).
Concerning the main outcome measures, we found 5 studies reported the EASI score. Using the random-effects model, the meta-analysis process revealed a highly significant increase in mean EASI score in the Biologic group compared to the placebo group (p=0.003). Which came in agreement with Bosma et al., [11] Boguniewicz et al., [2] Awosika et al., [12] Nettis et al. [1] and Simpson et al. [13]
Bosma et al. reported that, we found that women had significantly lower scores of EASI (>3.04 [SE, 0.75]) and IGA (>1.2 [SE, 0.32]) compared with men as a fixed effect over time during treatment, whereas the patients with skin type IV (n=19) had higher scores for EASI (12.9, P=.024), DLQI (12.56, P=.043), IGA (11.57, P=.0042) compared with skin type II (n=126). In addition, the use of concomitant immunomodulating systemic therapy resulted in lower estimated scores of EASI (change in score: -2.66, P=.0001), IGA (-0.73, P=.0046), and NRS mean pruritus past 7 days (>0.77, P=.0231) compared with absence of concomitant therapy. [11]
Boguniewicz et al. reported that two phases of 3 trials showed significantly greater improvement in the EASI score (≥75%) from baseline to week 16 in dupilumab-treated patients. Dupilumab helps with decreasing skin inflammation. Treatment with dupilumab was associated with major improvements in AD-associated symptoms, including pruritus and anxiety and depression. [2]
Awosika et al. reported that, in his M12 study, dupilumab showed significant improvement in the severity of AD in adults with moderate-to-severe disease. Dupilumab also helped improvement in multiple clinical measures in EASI and IGA scores, and pruritus numerical rating scale (NRS) score by day 85. Of note, 85% of patients on dupilumab achieved EASI 50 compared to 35% in the placebo group. [12]
Nettis et al. reported that, 543 patients with moderate-to-severe AD. Two patients (0.4%) discontinued treatment. The change from baseline to 16 weeks of treatment in the EASI score was -87.5 (p<0.001). EASI response rate reached 98.1% after 16 weeks. At 16 weeks, 93.0% of the patients had achieved an improvement in DLQI from baseline. [1]
Simpson et al. reported that endpoints were analyzed according to a prespecified hierarchy. The proportion of patients who had an improvement from baseline at week 16 of at least 75% on the Eczema Area and Severity Index (EASI-75) was a key secondary endpoint (and was identified as a co-primary endpoint by regulators in the European Union and Japan). [13] Our result came in disagreement with Drucker et al. [4]
Drucker et al. reported that, Dupilumab 300 mg every 2 weeks (the approved dosage for adults) was superior to placebo (mean difference, 11.3-point reduction. Several investigational medications demonstrated a reduction in EASI score compared with placebo, including baricitinib, 2 mg daily (mean difference, 5.6- point reduction, and 4 mg daily (mean difference, 5.2-point reduction. [4]
We found 4 studies reported the DLQI score, using the random-effects model, the meta-analysis process revealed a non-significant difference in mean DLQI score in the Biologic group compared to the placebo group (p>0.05). Which came in disagreement with Drucker et al., [4] Bosma et al., [11] Caputo et al. [14] and Fourzali et al. [15]
Drucker et al. reported that, Dupilumab, 300 mg every 2 weeks (mean difference, –4.8, and abrocitinib, 100mg daily (mean difference, –5.2) and 200 mg daily (mean difference, –4.9), were associated with clinically important differences in the DLQI score compared with placebo. [4]
Bosma et al. reported that baseline scores for POEM and DLQI were comparable or higher. After 12 to 24 weeks of treatment, we found similar scores of both investigator-reported as well as patient-reported outcomes. [11]
Caputo et al. reported that patients aged 11 to 16 years had a proportional improvement in the quality of life and clinical presentation. Two patients presented partial response in clinical picture and 60% of patients improved significantly according to SCORAD, with values - from 59, 42, 37 before the infusion to 22, 12, 19 after 6 months, respectively. [14]
Fourzali et al. reported that the symptoms of AD cause considerable stress, psychological effects, and burden on patients. Phase III trials with dupilumab confirmed what had been observed in earlier Phase trials, with clinically significant improvements in QoL scores. In the CHRONOS trial, significant improvements in DLQI scores were evident compared with placebo and were maintained through 52 weeks. [15]
We found 2 studies reported POEM score, using the randomeffects model, the meta-analysis process revealed a nonsignificant difference in mean POEM score in the Biologic group compared to the placebo group, which came in disagreement with Drucker et al., [4] Bosma et al. [11] and Awosika et al. [12]
Drucker et al. reported that Dupilumab, 300 mg every 2 weeks (mean difference, –7.5), and investigational drugs abrocitinib, 100 mg daily (mean difference, –7.6) and 200 mg daily (mean difference, –11.3), and up adacitinib, 15 mg daily (mean difference, –7.0) and 30 mg daily (mean difference, –10.7) were associated with clinically relevant improvements in the POEM score compared with placebo. [4]
Bosma et al. reported that they observed improvement of clinical signs (EASI, IGA), patient-reported symptoms (POEM, NRS pruritus), and quality of life (DLQI), in particular in the first 12 weeks of treatment. Estimated POEM score (0-28) decreased from 25.9 at baseline to 9 at 84 weeks. [11]
Awosika et al. reported that dupilumab significantly decreased patient-reported symptoms of AD, with improvement in sleep, anxiety, depression, and, therefore, the quality of life of subjects. [12-15]
Conclusion
To conclude, atopic dermatitis is a chronic, recurrent, inflammatory disease, characterized by intense itching and eczema. Patients may need immunobiological therapies to achieve proper disease control.
Competing Interests
The authors declare that they have no competing interests. All the listed authors contributed significantly to the conception and design of study, acquisition, analysis, and interpretation of data and drafting of the manuscript, to justify authorship.
REFERENCES
- Nettis E, Ferrucci SM, Ortoncelli M, Pellacani G, Foti C, Di Leo E, et al. Use of Dupilumab for 543 Adult Patients with Moderate-To-Severe Atopic Dermatitis: A Multicenter, Retrospective Study. J Investig Allergol Clin Immunol. 2020.
- Boguniewicz M, Fonacier L, Guttman-Yassky E, Ong PY, Silverberg J, Farrar JR. Atopic dermatitis yardstick: Practical recommendations for an evolving therapeutic landscape. Ann Allergy Asthma Immunol. 2018;120:10-22.
- Jeon C, Yan D, Nakamura M, Sekhon S, Bhutani T, Berger T, et al. Frequency and management of sleep disturbance in adults with atopic dermatitis: a systematic review. Dermatol Ther. 2017;7:349-364.
- Drucker AM, Ellis AG, Bohdanowicz M, Mashayekhi S, Yiu ZZ, Rochwerg B, et al. Systemic Immunomodulatory Treatments for Patients With Atopic Dermatitis: A Systematic Review and Network Meta-analysis. JAMA Dermatology 2020.
- Liberati A, Altman D, Tetzlaff J, Mulrow C, Gøtzsche P, Ioannidis J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions. Bmj 2009;339.
- Thaçi D, Simpson EL, Beck LA, Bieber T, Blauvelt A, Papp K, et al. Efficacy and safety of dupilumab in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical treatments: a randomised, placebo-controlled, dose-ranging phase 2b trial. The Lancet 2016;387:40-52.
- Simpson EL, Flohr C, Eichenfield LF, Bieber T, Sofen H, Taïeb A, et al. Efficacy and safety of lebrikizumab (an anti-IL-13 monoclonal antibody) in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical corticosteroids: a randomized, placebo-controlled phase II trial (TREBLE). J Am Acad Dermatol. 2018;78:863-871.
- Guttman-Yassky E, Silverberg JI, Nemoto O, Forman SB, Wilke A, Prescilla R, et al. Baricitinib in adult patients with moderate-to-severe atopic dermatitis: a phase 2 parallel, double-blinded, randomized placebo-controlled multiple-dose study. J Am Acad Dermatol. 2019;80:913-921.
- Silverberg JI, Pinter A, Pulka G, Poulin Y, Bouaziz J-D, Wollenberg A, et al. Phase 2B randomized study of nemolizumab in adults with moderate-to-severe atopic dermatitis and severe pruritus. J Allergy Clin Immunol. 2020;145:173-182.
- Simpson EL, Lacour J-P, Spelman L, Galimberti R, Eichenfield LF, Bissonnette R, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis and inadequate response to topical corticosteroids: results from two randomized monotherapy phase III trials. Br J Dermatol. 2020.
- Bosma AL, de Wijs LE, Hof MH, van Nieuwenhuizen BR, Gerbens LA, Middelkamp-Hup MA, et al. Long-term effectiveness and safety of treatment with dupilumab in patients with atopic dermatitis: results of the TREAT NL (TREatment of ATopic eczema, the Netherlands) registry. J Am Acad Dermatol. 2020.
- Awosika O, Kim L, Mazhar M, Rengifo-Pardo M, Ehrlich A. Profile of dupilumab and its potential in the treatment of inadequately controlled moderate-to-severe atopic dermatitis. Clin Cosmet Investig. 2018;11:41.
- Simpson JC, Bao X, Agarwala A. Enhanced Recovery after Surgery (ERAS) for Colorectal Surgery: Pain Management in Enhanced Recovery after Surgery (ERAS) Protocols. Clin Colon Rectal Surg. 2019;32:121.
- Caputo MM, Silva PP, Pittelkow P, Bastos PGA, Guerzet P, Japiassú LG, et al. Gamaglobulin infusion in patients with severe atopic dermatitis. J Allergy Clin Immunol. 2019;143:AB134.
- Fourzali K, Golpanian RS, Yosipovitch G. Dupilumab use in atopic dermatitis and beyond in skin diseases. Immunotherapy 2020.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.