Prevalence of COPD Concerning Severity of COVID-19 Infection: A Short Systematic Review and Metaanalysis
2 Medical Intern, Taif University, Taif, Saudi Arabia
3 Medical Resident, King Saud Medical City, Riyadh, Saudi Arabia
4 Medical Intern, Princes Sattam Bin Abdulaziz University, AlKharj, Saudi Arabia
5 5th Year Medical Student, AlQassim University, AlQassim, Saudi Arabia
6 Medical Intern, Ibn Sina National College, Jeddah, Saudi Arabia
7 Medical Intern, Northen Border University, Arar, Saudi Arabia
8 Family Medicine Resident, Primary Health Care Center, Jeddah, Saudi Arabia
9 Medical Intern, King Abdulaziz University, Jeddah, Saudi Arabia
10 Medical Intern, King Khalid University, Abha, Saudi Arabia
Citation: Alluhaymid YM, et al. Prevalence of COPD Concerning Severity of COVID-19 Infection: A Short Systematic Review and Meta-analysis. Ann Med Health Sci Res. 2020;10:1095-1099.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Background: Concerning COVID-19, levels of angiotensin-converting enzyme 2 (ACE2), the said host receptor of the virus responsible for COVID-19 (intense acute respiratory syndrome coronavirus 2; SARS-CoV-2), were located to be elevated in patients with COPD. But, early character COVID-19 studies have not always pronounced a drastically better fee of severe disease in COPD sufferers. Aim: This work aims to determine the prevalence of COPD concerning the severity of COVID-19 infection. Materials and Methods: A systematic search was performed over different medical databases to identify Internal Medicine studies, which studied the outcome of the COPD group versus the Non-COPD group of COVID-19 patients. Using the metaanalysis process, either with fixed or random-effects models, we conducted a metaanalysis on the prevalence of severe cases as a primary outcome, and on mortality rate as a secondary outcome. Results: Eleven studies were identified involving 146666 patients, 2764 in the COPD group, and 143902 in the Non-COPD group. The metaanalysis process revealed that the pooled prevalence of severity among COVID-19 patients was (28.7%), and there is a significant increase in COVID-19 severe cases in the COPD group (OR=3.46, P=0.042). The pooled mortality rate among COVID-19 patients was (16.7%), and there is a highly significant increase in mortality in COPD group (OR=3.75, P<0.001). Conclusion: To conclude, COVID-19 is an emerging disease all over the world and spreading at an unpredicted rate, resulting in significant influences on global economies and public health. The present review assessed the risk factors related to the outcomes of SARS-CoV-2 infections which were included old age, male gender, and obesity, associated co-morbidities in COPD, which increase the mortality and severity of COVID-19. mp3download.link Best YouTube to MP3 converter. Download MP3 from YouTube for Free. one would honestly have expected more on the track https://www.mp3-go.net Download Mp3 songs for free Given the legendary pedigree of the man behind the sound downloadmp3-gratis.biz Download mp3 songs online at Mp3 Converter, watch high quality online music videos download-mp3gratis.me watch and download free songs of the highest quality. Listen to songs online here comfortably without any annoying advertisements. metrolagu.site Easy to use and free MP3 downloader. YouTube To MP3 download in seconds using the best YouTube to MP3 converter. YouTube To Mp3 Get the latest song by simply typing the latest artist or song title in the Search menu. Mp3 file format with 128 - 320 Kbps bitrate converted from YouTube videos. read at this blog All those artist performances are still available on YouTube today find more here
Keywords
COPD; Severity COVID-19; Mortality
Introduction
The coronavirus disorder 2019 (COVID-19) outbreak is now pandemic, straining medical infrastructure, personnel, and resources in much of Europe, the center East, and North the USA, with significant results for clinical control, including rationing of care. Current facts suggest that 14% of COVID- 19eassociated pneumonia instances are severe, and five% of inflamed patients require intensive care. Mortality rates in severe and critically ill sufferers are staggering, with the disorder being fatal in approximately two thirds. [1]
The COVID-19 pandemic has continued to spread globally, and even though the majority is asymptomatic or only display a mild, flu-like illness, a significant percentage develops an excessive response. Intense COVID-19 can result in fatal complications, which include acute respiration distress syndrome, multi-organ failure, and death. The question as to why a few individuals become severely ill, whilst others do no longer, remains a puzzle to be solved. it’s far therefore of great importance to discover risk factors or comorbidities associated with severe COVID-19 to protect the susceptible and allow for prudent resource allocation. [2]
Although the overall fatality rate of COVID-19 is low older adults and patients with comorbidities are much more likely to have an extreme disease and subsequent mortality. The most typically reported non-communicable sicknesses that have been proven to predict bad prognosis in patients with COVID-19 include diabetes mellitus (DM), hypertension, cerebrovascular disease, coronary artery disease (CAD), and chronic obstructive pulmonary disease (COPD). [3]
Chronic Obstructive Pulmonary Disease (COPD) is related to an increased threat of morbidity and mortality in networkacquired pneumonia (CAP). Changes in local/systemic inflammatory reaction, impaired host immunity, microbiome imbalance, persistent mucus production, structural damage, and use of inhaled corticosteroids have been hypothesized to make contributions to such hazards. Concerning COVID-19, levels of angiotensin-converting enzyme 2 (ACE2), the said host receptor of the virus responsible for COVID-19 (intense acute respiratory syndrome coronavirus 2; SARS-CoV-2), were located to be elevated in patients with COPD. But, early character COVID-19 studies have not always pronounced a drastically better fee of severe disease in COPD sufferers. [4]
This work aims to determine the prevalence of COPD concerning the severity of COVID-19 infection.
Literature Review
Our review came following the (PRISMA) statement guidelines. [5]
Study eligibility
The included studies should be in English, a journal published article, and a human study describing COVID-19 patients. The excluded studies were non-English or animal studies.
Study identification
Basic searching was done over the PubMed, Cochrane library, and Google scholar using the following keywords: COPD, severity COVID-19, mortality.
Data extraction and synthesis
RCTs, clinical trials, and comparative studies, which studied the outcome of the COPD group versus the Non-COPD group of COVID-19 patients, will be reviewed. Outcome measures included the prevalence of severe cases (as a primary outcome), and mortality rate (as a secondary outcome).
Study selection
We found 94 records, 34 excluded based on title and abstract review; 60 articles are searched for eligibility by full-text review; 22 articles cannot be accessed; 16 studies were reviews and case reports; 11 were not describing functional outcome; leaving 11 studies that met all inclusion criteria.
Statistical methodology
The pooling of data, Proportions (%), Odds Ratios (ORs), with 95% confidence intervals (CI) were done, using MedCalc ver. 18.11.3 (MedCalc, Belgium). According to heterogeneity across trials using the I2-statistics; a fixed-effects model or randomeffects model were used in the meta-analysis process.
Results
The included studies were published in 2020. Regarding the type of included studies, all studies were retrospective [Table 1]. Regarding patients’ characteristics, the total number of patients in all the included studies was 146666 patients, 2764 in the COPD group, and 143902 in the Non-COPD group [Table 1].
Table 1: Patients and study characteristics.
| N | Author | Type of study | Number of patients | Age (average years) | Male patients | ||
|---|---|---|---|---|---|---|---|
| Total | COPD group | Non-COPD group | |||||
| 1 | L. Chen et al. [6] | Retrospective | 1859 | 61 | 1798 | 59 | 934 |
| 2 | M. Chen et al. [7] | Retrospective | 123 | 6 | 117 | 62.5 | 61 |
| 3 | Gao et al. [8] | Retrospective | 43 | 8 | 35 | 44 | 26 |
| 4 | Guan et al. [9] | Retrospective | 1099 | 12 | 1087 | 46.7 | 459 |
| 5 | Li et al. [10] | Retrospective | 102 | 2 | 100 | 62 | 59 |
| 6 | Liu et al. [11] | Retrospective | 78 | 2 | 76 | 42.7 | 39 |
| 7 | Parra-Bracamonte et al. [12] | Retrospective | 142690 | 2655 | 140035 | 50 | 79280 |
| 8 | Yang et al. [13] | Retrospective | 52 | 4 | 48 | 59.7 | 35 |
| 9 | Zhang, Cao, et al. [14] | Retrospective | 289 | 6 | 283 | 57 | 154 |
| 10 | Zhang, Dong, et al. [15] | Retrospective | 140 | 2 | 138 | 56.3 | 71 |
| 11 | Zhou et al. [16] | Retrospective | 191 | 6 | 185 | 56.3 | 119 |
#Studies arranged alphabetically.
The mean age of all patients was (54.2 years), with 81237 (55.4%) male patients [Table 1]. [6-16]
A meta-analysis study was done on 11 studies that described and compared the 2 different groups of patients; with an overall number of patients (N=146666) [Table 2]. [6-16]
Table 2: Summary of outcome measures in all studies.
| N | Author | Primary outcome | Secondary outcome | ||
|---|---|---|---|---|---|
| Severe COVID-19 cases | Mortality rate | ||||
| COPD group | Non-COPD group | COPD group | Non-COPD group | ||
| 1 | L. Chen et al. [6] | --- | --- | 12 | 196 |
| 2 | M. Chen et al. [7] | --- | --- | 3 | 28 |
| 3 | Gao et al. [8] | 3 | 12 | --- | --- |
| 4 | Guan et al. [9] | 7 | 60 | --- | --- |
| 5 | Li et al. [10] | --- | --- | 1 | 14 |
| 6 | Liu et al. [11] | 1 | 10 | --- | --- |
| 7 | Parra-Bracamonte et al. [12] | --- | --- | 871 | 16001 |
| 8 | Yang et al. [13] | 2 | 30 | --- | --- |
| 9 | Zhang, Cao, et al. [14] | 2 | 76 | 3 | 46 |
| 10 | Zhang, Dong, et al. [15] | 2 | 56 | --- | --- |
| 11 | Zhou et al. [16] | 4 | 50 | 4 | 50 |
Each outcome was measured by:
Odds Ratio (OR)
• For COVID-19 severity and COPD.
• For mortality and COPD.
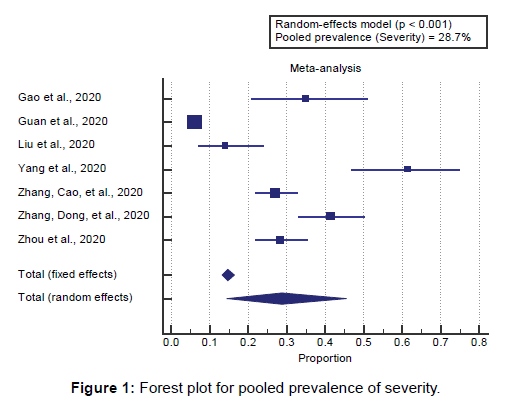
Concerning the primary outcome measure, we found 7 studies reported COVID-19 severity with a total number of patients (N=1892). I2 (inconsistency) was 97.6% with a highly significant Q test for heterogeneity (p<0.0001), so the randomeffects model was carried out. Using the random-effects model, the pooled prevalence of severity among COVID-19 patients was (28.7%) [Figure 1].
Using the random-effects model, the meta-analysis process revealed a significant increase in COVID-19 severe cases in the COPD group compared to the Non-COPD group (OR=3.46, P=0.042) [Figure 2].
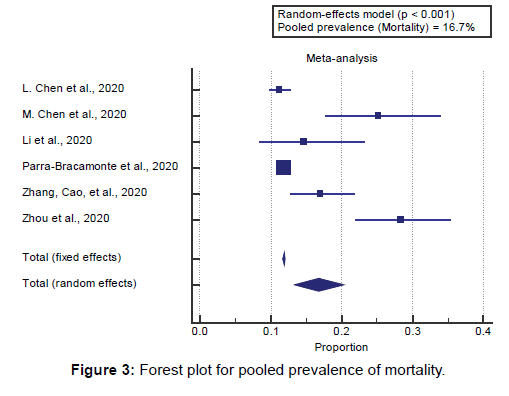
Concerning the secondary outcome measures, we found 6 studies reported mortality rates with a total number of patients (N=145254). I2 (inconsistency) was 91.3% with a highly significant Q test for heterogeneity (p<0.0001), so the randomeffects model was carried out.
Using the random-effects model, the pooled mortality rate among COVID-19 patients was (16.7%) [Figure 3].
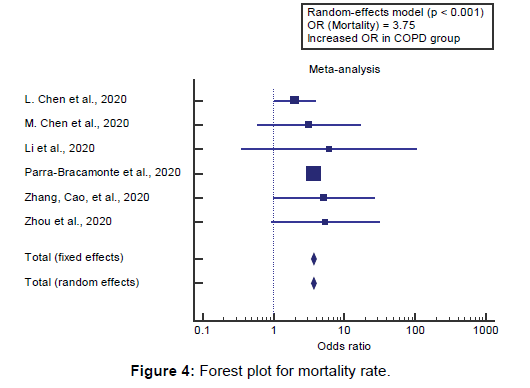
Using the random-effects model, the meta-analysis process revealed a highly significant increase in mortality in COPD group compared to Non-COPD group (OR=3.75, P<0.001) [Figure 4].
Discussion
This work aims to determine the prevalence of COPD concerning the severity of COVID-19 infection.
The included studies were published in 2020. Regarding the type of included studies, all studies were retrospective.
Regarding patients’ characteristics, the total number of patients in all the included studies was 146666 patients, 2764 in the COPD group, and 143902 in the Non-COPD group. The mean age of all patients was (54.2 years), with 81237 (55.4%) male patients. A meta-analysis study was done on 11 studies that described and compared the 2 different groups of patients; with an overall number of patients (N=14666). Concerning the primary outcome measure, we found 7 studies reported COVID-19 severity with a total number of patients (N=1892).
Using the random-effects model, the pooled prevalence of severity among COVID-19 patients was (28.7%). Using the random-effects model, the meta-analysis process revealed a significant increase in COVID-19 severe cases in the COPD group compared to the Non-COPD group (OR=3.46, P=0.042, which came in agreement with Alqahtani et al., [17] Zhao et al., [18] Pranata et al., [2] Cox et al., [19] and Pal & Bhadada. [3]
Alqahtani et al., reported that, overall, 123 abstracts were screened and 61 full-text manuscripts have been reviewed. A total of 15 studies met the inclusion standards, which included a total of 2473 confirmed COVID-19 patients. All studies had been included within the meta-analysis. The crude case fatality rate of COVID-19 became 7.4%. The pooled occurrence rates of COPD patients and smokers in COVID-19 cases had been 2% and 9% respectively. COPD sufferers were at a better hazard of more severe disorder (risk of severity=63%, (22/35). [17]
Zhao et al., reported that a total of 10 studies were included inside the evaluation to decide the effect of pre-present COPD on the severity of COVID-19, as 1 of the research did not record on the prevalence of COPD of their patient population. The pooled odds ratio (OR) of COPD for the development of severe COVID-19 is which suggests that the presence of COPD is associated with an almost fourfold better risk of growing extreme COVID-19 (fixed-effects version; OR=4.38; heterogeneity among the different studies being mild (I2=41%; P=0.08). Sensitivity analysis showed that the results had been not affected by any individual study. [18]
Pranata et al., reported that, COPD showed higher risk for composite poor outcome (OR 5.01, P<0.001; I2 0%, P=0.98) [Figure 1]. Subgroup analysis of COPD patients showed a higher mortality (OR 4.36, P=0.009; I2 0%, P=0.88), greater likelihood of having severe COVID-19 (OR 4.62, P<0.001; I2 0%, P=0.78), ICU care (OR 8.33, P=0.03; I2 0%, P=0.89) and disease progression (OR 8.42, P=0.01; I2 0%, P=0.84). [2]
Cox et al., reported that chronic obstructive pulmonary sickness (COPD) is a hazard factor for severe COVID-19 disorder and many patients with COPD may have underlying chronic bacterial infections before severe acute respiration syndrome coronavirus 2 (SARS-CoV-2) infections, however, this important fact isn’t always being pronounced. More statistics on co-infections are urgently required to establish their importance in COVID-19 severity and mortality. [19]
Pal & Bhadada, reported that even though the overall fatality charge of COVID-19 is low older adults and patients with comorbidities are much more likely to have a severe ailment and next mortality. The most normally reported non-communicable diseases which have been proven to predict poor prognosis in patients with COVID-19 to include diabetes mellitus (DM), hypertension, cerebrovascular disease, coronary artery disease (CAD) and chronic obstructive pulmonary disease (COPD). [3] Our result came in disagreement with Zhang et al. [14]
Zhang et al., reported that, because of these results, smoking should not be considered a preventive measure for COVID-19 and as a public health issue should be discouraged at all times. Although smoking is a major cause of the chronic obstructive pulmonary disease (COPD), a recent study reported that COPD did not increase the risk of COVID-19 patients requiring admission to the intensive care unit (ICU). [14]
Concerning the secondary outcome measures, we found 6 studies reported mortality rates with a total number of patients (N=145254).
Using the random-effects model, the pooled mortality rate among COVID-19 patients was (16.7%). Using the randomeffects model, the meta-analysis process revealed a highly significant increase in mortality in COPD group compared to Non-COPD group (OR=3.75, P<0.001), which came in agreement with Alqahtani et al., [17] Aly et al., [20] Lippi & Henry, [4] Zhao et al., [18] Leung et al., [21] and Grasselli et al. [22]
Alqahtani et al., reported that COPD patients were at a higher risk of more severe disease (risk of severity=63%, (22/35) compared to patients without COPD 33.4% (409/1224) [calculated RR, 1.88]. This was associated with higher mortality (60%). [17]
Aly et al., reported that clinical characteristics of 25 cases died with COVID-19. The clinical profile of these patients showed that the most important risk factors for death in these patients represented in, age, and underlying diseases. Regarding the underlying illnesses associated with death, the most recorded one was chronic hypertension than diabetes mellitus, chronic cardiac diseases, cerebral infarction, kidney disease, chronic obstructive pulmonary disease. [20]
Lippi & Henry, reported that Chronic Obstructive Pulmonary Disease (COPD) is associated with an increased risk of morbidity and mortality in community-acquired pneumonia (CAP). Alterations in local/systemic inflammatory response, impaired host immunity, microbiome imbalance, persistent mucus production, structural damage, and use of inhaled corticosteroids have been hypothesized to contribute to such risk. [4]
Zhao et al., reported that only two of the included studies reported the association between death and pre-existing COPD. Death was reported in 6 of 10 (60%) of patients with COPD and 80 of 233 (34.3%) of non-COPD patients. The pooled OR of COPD for death was 1.93 however, the heterogeneity in this analysis (I2=61%; P=.11) was quite high. [18]
Leung et al., reported that there is a global outbreak of COVID-19 coronavirus. Although most patients inflamed and identified with CVOID-19 sickness have moderate signs and symptoms, approximately 20% of people have established extreme or severely severe sickness including signs and symptoms of pneumonia, breathing failure, septic shock, and multi-organ failure. The estimated case-fatality fee is 1-2%. Importantly, almost all deaths have taken place in those with significant underlying continual sicknesses such as COPD and cardiovascular sicknesses. The reason for this observation is largely unknown. [21]
Grasselli et al., reported that, at multivariable analysis, a 10- year increase in age (HR, 1.75) and male sex (HR, 1.57) were significantly associated with mortality. Among comorbidities, history of chronic obstructive pulmonary disease (HR, 1.68), hypercholesterolemia (HR, 1.25), and diabetes (HR, 1.18) was significantly associated with mortality. [22]
Conclusion
To conclude, COVID-19 is an emerging disease all over the world and spreading at an unpredicted rate, resulting in significant influences on global economies and public health. The present review assessed the risk factors related to the outcomes of SARS-CoV-2 infections which were included old age, male gender, and obesity, associated co-morbidities in COPD, which increase the mortality and severity of COVID-19.
Competing Interests
The authors declare that they have no competing interests. All the listed authors contributed significantly to the conception and design of study, acquisition, analysis, and interpretation of data and drafting of the manuscript, to justify authorship.
REFERENCES
- Xie J, Covassin N, Fan Z, Singh P, Gao W, Li G, et al. Association between hypoxemia and mortality in patients with COVID-19. Mayo Clinic Proceedings, Elsevier; 2020.
- Pranata R, Soeroto AY, Ian H, Lim MA, Santoso P, Permana H. Effect of chronic obstructive pulmonary disease and smoking on the outcome of COVID-19. Int J Tuberc Lung Dis 2020;10.
- Pal R, Bhadada SK. COVID-19 and non-communicable diseases. Postgraduate Medical Journal 2020.
- Lippi G, Henry BM. Chronic obstructive pulmonary disease is associated with severe coronavirus disease 2019 (COVID-19). Respir Med. 2020;167:105941.
- Liberati A, Altman D, Tetzlaff J, Mulrow C, Gøtzsche P, Ioannidis J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions. Bmj 2009;339.
- Chen L, Yu J, He W, Chen L, Yuan G, Dong F, et al. Risk factors for death in 1859 subjects with COVID-19. Leukemia 2020;34:2173–2183.
- Chen M, Fan Y, Wu X. Clinical Characteristics And Risk Factors For Fatal Outcome in Patients With 2019-Coronavirus Infected Disease (COVID-19) in Wuhan, China (2/27/2020). Publisher Full Text 2020.
- Gao Y, Li T, Han M, Li X, Wu D, Xu Y, et al. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J Med Virol. 2020.
- Guan W, Ni Z, Hu Y, Liang W, Ou C, He J, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720.
- Li K, Chen D, Chen S. Radiographic findings and other predictors in adults with COVID-19. medRxiv; 2. Epub Ahead of Print 2020;27.
- Liu W, Tao Z-W, Wang L, Yuan M-L, Liu K, Zhou L, et al. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J. 2020.
- Parra-Bracamonte GM, Lopez-Villalobos N, Parra-Bracamonte FE. Clinical characteristics and risk factors for mortality of patients with COVID-19 in a large dataset from Mexico. Ann Epidemiol. 2020.
- Yang X, Yu Y, Xu J, Shu H, Liu H, Wu Y, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020.
- Zhang J, Cao Y, Tan G, Dong X, Wang B, Lin J, et al. Clinical, radiological and laboratory characteristics and risk factors for severity and mortality of 289 hospitalized COVID-19 patients. Allergy 2020.
- Zhang J, Dong X, Cao Y, Yuan Y, Yang Y, Yan Y, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy 2020.
- Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The Lancet 2020.
- Alqahtani JS, Oyelade T, Aldhahir AM, Alghamdi SM, Almehmadi M, Alqahtani AS, et al. Prevalence, severity and mortality associated with COPD and smoking in patients with COVID-19: A rapid systematic review and meta-analysis. PloS One 2020;15:e0233147.
- Zhao Q, Meng M, Kumar R, Wu Y, Huang J, Lian N, et al. The impact of COPD and smoking history on the severity of COVID-19: a systemic review and meta-analysis. Journal of Medical Virology 2020.
- Cox MJ, Loman N, Bogaert D, O’grady J. Co-infections: potentially lethal and unexplored in COVID-19. The Lancet Microbe 2020;1:e11.
- Aly MH, Rahman SS, Ahmed WA, Alghamedi MH, Al Shehri AA, Alkalkami AM, et al. Indicators of critical illness and predictors of mortality in COVID-19 patients. Infect Drug Resist. 2020;13:1995.
- Leung JM, Yang CX, Tam A, Shaipanich T, Hackett T-L, Singhera GK, et al. ACE-2 expression in the small airway epithelia of smokers and COPD patients: implications for COVID-19. Eur Respir J. 2020;55.
- Grasselli G, Greco M, Zanella A, Albano G, Antonelli M, Bellani G, et al. Risk factors associated with mortality among patients with COVID-19 in Intensive Care Units in Lombardy, Italy. JAMA Internal Medicine 2020.





 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.