Quantitative Assessment of the Active Ingredient of Artemether Injection Products Before and After Induced Degradation
Received: 15-Apr-2024, Manuscript No. amhsr-24-137763; Editor assigned: 17-Apr-2024, Pre QC No. amhsr-24-137763 (PQ); Reviewed: 01-May-2024 QC No. amhsr-24-137763 ; Revised: 08-May-2024, Manuscript No. amhsr-24-137763 (R); Published: 15-May-2024
Citation: Bulus D. Quantitative Assessment of the Active Ingredient of Artemether Injection Products Before and After Induced Degradation. Ann Med Health Sci Res. 2024;14:973-980.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
The induced degradation profile of artemether injection products was accomplished with chromatographic separation using C18 column (25 cm × 4.6 mm) material 5 μm size with mobile phase combination of acetonitrile and water in the ratio of 62:38, a flow rate of 1.5 mL/min, and detection wavelength of 216 nm. The regression equation was found to be y=2.026 × +0.177 with R^2 value of 0.9976, and an accuracy of 0.42% RSD. The assay specification limit for interday precision was found to be 1.26% RSD while the intra-day was 0.83% RSD establishing the validity of the method for linearity, accuracy, and precision respectively. The percent contents of the active pharmaceutical ingredients of each sample before degradation ranges from 92.8% to 129.3% resulting in five samples (C, D, I, J, and M) having percent contents that complies with the international pharmacopeia standard (IPh) of 90.0 to 110.0%. It was found that ten (10) of the samples (A, B, E, F, G, H, K, L, N, and O) had higher percent artemether contents out of the fifteen (15) analyzed samples. The results of forced degradation in photolytic environment at 366 nm ranges from 0.4 to 34.9% indicating that the samples were more stable in the photolytic environment, with the exception of sample N and K with 34.9% and 23.8% percent degradation. The result of the degradation pattern in thermal environment at 70℃ had a range of 18.9% to 54.4%. Furthermore the results of forced degradation in acidic environment (5.0 NHCl for 30 min at 70℃) ranges from 14.0% to 85.5% indicating that the samples were unstable in the acidic environment, with the exception of sample M with 14.0% percent degradation. Also the result of the degradation pattern in basic environment (5.0 N NaOH for 30 min at 70℃) had a range of 29.9 to 62.7%. All the analyzed samples with the exception of sample M in acidic and basic environment were found to be above the 20% maximum acceptance criteria of the International Conference on Harmonization (ICH). The result of the statistical analysis of the samples shows a significant difference at p<0.05 across each degradation environment.
Keywords
Artemether; Induced degradation; Environment; Percent content; Photolytic; Stability; Thermal.
Introduction
Pharmaceuticals are preparations or formulations that contain approved predetermined amount of one or more active chemical substance intended for medical diagnosis, cure, treatment or prevention of disease [1]. When active pharmaceutical ingredients are not present in the approved amount for any drug, it is a counterfeit or a substandard drug. This may be because the medication does not contain the predetermined active ingredient, or it has undergone degradation. These counterfeit drugs or degraded drugs can lead to treatment failure, increased toxicity, and increased drug resistance to malaria parasite which may lead to death [2].
Malaria is a parasitic disease caused by protozoa of the genus plasmodium that infect and destroy the red blood cells leading to fevers, severe anemia, and may culminate to cerebral malaria. There are four main species of plasmodium that infect humans with malaria. The P. falciparum, P. Vivax, P. malariae and P. ovale. This plasmodium can adapt over time and become resistant to previously effective drugs. The adaptation may be due to low active ingredient or low quality of active ingredient due to poor formulations or product degradations [3].
Artemether is a potent and rapidly acting antimalaria agent which is enlisted in World Health Organization (WHO) list of essential medicines for the treatment of severe multiresistant malaria (World Health Organization). It is used for the treatment of malaria caused by multi-drug resistant strains of Plasmodium falciparum and Plasmodium vivax; it is effective against the blood flukes. Artemether is a methyl ether derivative of artemisinin, which is a peroxide-containing lactone isolated from the anti-malarial plant Artemisia annua. It is also known as dihydroartemisinin methyl ether, its chemical nomenclature is (+)-(3-alpha,5a-beta,6-beta,8a-beta,9-alpha,12-beta,12aR)- decahydro-10-methoxy-3,6,9-trimethyl-3,12-epoxy-12Hpyrano( 4,3-j)-1,2-benzodioxepin. It is a relatively lipophilic and unstable drug, which acts by creating reactive free radicals in addition to affecting the membrane transport system of the plasmodium organism [1] (Figure 1).
Stability indicating method
Forced degradation of a new drug substance or drug product at conditions more severe than accelerated conditions is required to demonstrate the specificity of stability indicating methods and provides an insight into degradation pathways and degradation products of the drug substance which helps in elucidation of the structure of the degradation products. These studies show the chemical behavior of the molecule which in turn helps in the development of the formulation and also packaging requirements. Chemical stability of pharmaceutical molecules is a matter of great concern as it affects the safety, purity and efficacy of the drug product. The FDA and ICH guidelines state the requirement of stability testing data to understand how the quality of a drug substance and drug product changes with time under the influence of various environmental factors [4].
Forced degradation is a process that involves degradation of drug products and drug substances at conditions more severe than accelerated conditions and thus generates degradation products that can be studied to determine the stability of the molecule [4]. The samples generated from forced degradation can be used to develop the stability indicating method which can be applied later for the analysis of samples generated from accelerated and longer stability studies [4].
Condition for forced degradation
It is very important to know when to perform forced degradation studies for the development of new drug substance and new drug product. FDA guidance states that stress testing should be performed in phase III of regulatory submission process. Stress studies should be done in different pH solutions, in the presence of oxygen and light, and at elevated temperatures and humidity levels to determine the stability of the drug substance [5]. An early stress study gives timely recommendations for making improvements in the manufacturing process and proper selection of stability-indicating analytical procedures [6].
Limits for degradation
Degradation of drug substances between 5% and 20% has been accepted as reasonable for validation of chromatographic assays [7]. Some pharmaceutical scientists think 10% degradation is optimal for use in analytical validation for small pharmaceutical molecules for which acceptable stability limits of 90% of label claim is common [8].
No such limits for physiochemical changes, loss of activity or degradation during shelf life have been established for individual types or groups of biological products. The study can be terminated if no degradation is seen after drug substance or drug product has been exposed to stress conditions other than those conditions mentioned in an accelerated stability protocol [4].
Selection of degradation conditions
Forced degradation is carried out to produce representative samples for developing stability-indicating methods for drug substances and drug products. The choice of stress conditions should be consistent with the product's decomposition under normal manufacturing, storage, and use conditions which are specific in each case [9].
There is no specification in regulatory guidelines about the conditions of pH, temperature and specific oxidizing agents to be used. The design of photolysis studies is left to the applicant's discretion although Q1B specifies that the light source should produce combined visible and ultraviolet (UV, 320-400 nm) out puts, and that exposure levels should be justified [8,10]. Some scientists have found it practical to begin with extreme conditions such as 80℃ or even higher temperatures and testing at shorter multiple time points, so that the rate of degradation can be evaluated [11].
Selection of drug concentration
It is recommended that the studies should be initiated at a concentration of 1 mg/mL [12]. It is usually possible to get even minute decomposition products in the range of detection. It is also suggested that some degradation studies should be done at a concentration which the drug is expected to be present in the final formulations [13,14].
Materials and Methods
Description of sample collection and reagent
Fifteen samples of good quality Artemether injection that have the mandatory NAFDAC registration number as well as other statutory features, were procured from reputable pharmaceutical stores in Kaduna Metropolis in in Kaduna state, Nigeria for the study, while the Artemether United State Pharmacopeia (USP) reference standard was procured from USA; Milli Q water (HPLC grade); All chemicals and reagents used were of analytical grade. Acetonitrile (HPLC grade), sodium hydroxide, hydrochloric acid, and hydrogen peroxide, all reagents and chemicals are from Merck KGaA Germany.
List of apparatus and equipment
The HPLC analysis was accomplished using Ultimate 300 high pressure liquid chromatography System, Octadecylsilane C18 column (25 mm × 4.6 mm) analytical column reversed–phase material of 5 μ size with variable wavelength detector.
Chromatographic conditions and mobile phase
For good separation of Artemether reverse phase C18 column (250 mm × 4.6 mm) 5 μm was used with mobile phase made of Acetonitrile: Water 62:38 at a flow rate of 1.5 mL/min with a wavelength detection set at 216 nm in the UV spectrophotometer a component of the HPLC [15].
Quality assurance
Preparation of the standard stock solution: Standard stock solution of Artemether was prepared by dissolving 16 mg of Artemether Reference Standard in 10 mL volumetric flask with 7 mL mobile phase. The resulting solution was sonicated to dissolve completely and the Artemether reference standard and made up to volume with the same diluents (mobile phase) to get concentration of 1.6 mg/mL of Stock standard solution. From this resulting solution, 1 mL of the solution was pipetted out into a 10 mL volumetric flask and diluted up to the mark with diluents (mobile phase) to get a working standard solution of 0.16 g/mL concentration. The resulting solution was transferred into amber vials for analysis in the HPLC System [15].
System suitability test: The standard solution which was prepared as per the procedure and was injected six-times, injections response of the standard drug was used to determine the relative standard deviation in percentage (%RSD) [4].
Accuracy: The accuracy of an analytical method validation is the closeness of test results obtained by that method to the true value (Standard value) using known quantity of Artemether standard at 50%, 100%, and 150% of assay specification limit. The analysis was done in triplicate for each level [4].
Precision: The system precision of the method was verified by six replicate injections of standard solution containing Artemether standard. The precision for repeatability (intra-day and inter-days precision) was carried out six times in a day to record any intra-day variations in the results [4].
Robustness: Robustness of an analytical procedure measures the estimation of the capacity the process to remain unaffected by minor, deliberate variation in the method parameters [4].
Linearity and range: Linearity of the method was studied by injecting six concentrations of artemether standard prepared and calibration curves were constructed by plotting peak area against the respective concentrations [4].
Limit of detection and limit of quantitation: Sensitivity of the proposed method was estimated in terms of Limit of Detection (LOD) and Limit of Quantitation (LOQ). The LOD and LOQ was calculated according to the formula given by the ICH guidelines as described below,
LOD is calculated from the formula:
LOD = (3.3 σ)/S
LOQ is calculated from the formula:
LOQ = (10 σ)/S
Where:
σ=Standard deviation of the response of calibration curve.
S=Slope of the calibration curve [4].
Preparation of the sample solution before degradation: 2 mL of the artemether injection of 80 mg strength was transferred into a 50 mL volumetric flask, and 25 mL of the mobile phase was added, mixed and made up to mark with the mobile phase and sonicated for 15 min (1.6 mg/mL). 1 mL of this solution was transferred into 10 mL volumetric flask and made up to volume with the mobile phase to obtain a solution with 0.16 mg/mL concentration. The resulting solution was transferred into amber vials for analysis [15].
Forced degradation of the drugs samples: Sample solutions containing 0.16 mg/mL Artemether was prepared in the diluents. This solution was used for forced degradation the average peak area of artemether sample after analysis was recorded to study the degradation products of the drug in the following conditions. 20 μL of the standard and sample solutions, before they are subjected to degradation and after they are subjected to the various degradation environments, were injected into the chromatographic system and the area for the artemether peak response was measured and the percentage content calculated using the relationship.
Cu=Ru/Rs × Cs
% Content of artemether=Ru/Rs × Cs/Cu × 100
Where:
Ru=peak response of sample
Rs=peak response of standard
Cs=concentration of Standard
Cu=concentration of sample [15].
Results and Discussion
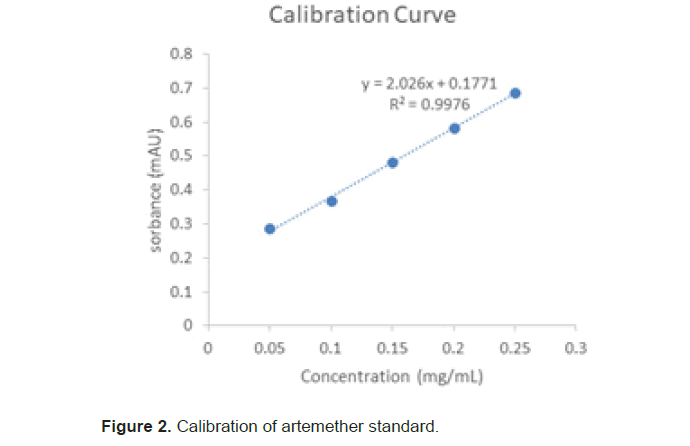
The results obtained from the analysis were treated to obtain the system suitability, accuracy, precision, robustness linearity, range limit of detection limits of quantification percent relative standard deviation of the instrument. The percentage content of the active pharmaceutical ingredient in the drug products before and after degradation was determined. After degradation the content of the active pharmaceutical ingredient responsible for the absorbance that produces the response was the active pharmaceutical ingredient remaining in the drug sample after degradation in the different environmental conditions that the samples were subjected to. The (Tables 1-7), (Figure 2) below contain the respective information of the results of analysis [16,17].
| Accuracy | |||
| Concentration (mg/mL) | 0.08 | 0.16 | 0.24 |
| Mean ± STD | 0.1313 ± 0.0006 | 0.6707 ± 0.0047 | 0.6943 ± 0.0047 |
| %RSD | 0.46 | 0.71 | 0.68 |
Table 1: Accuracy of the instrument in measuring the absorbance of Artemether Standard.
| S/No. | Retention Time (min) | Standard peak response (mAU*min) | Concentration (mg/mL) |
|---|---|---|---|
| 1 | 5.95 | 0.661 | 0.16 |
| 2 | 5.95 | 0.665 | 0.161 |
| 3 | 5.95 | 0.658 | 0.159 |
| 4 | 5.96 | 0.66 | 0.16 |
| 5 | 5.95 | 0.66 | 0.16 |
| 6 | 5.95 | 0.657 | 0.159 |
| Mean | 5.952 | 0.66 | 0.16 |
| STDEV | 0.004 | 0.003 | |
| %RSD | 0.069 | 0.422 |
Table 2: System suitability of the instrument in measuring the concentration of Artemether Standard.
| Precision | Inter day | Intra day | |
|---|---|---|---|
| Mean ± STDEV | 0.7169 ± 0.009 | Mean ± STDEV | 0.6722 ± 0.006 |
| %RSD | 1.26 | %RSD | 0.83 |
Table 3: Precision of the instrument in measuring the absorbance of Artemether Standard.
| Robustness of developed and reference methods | |||
|---|---|---|---|
| Parameter | Condition | Mean peak area SD | %RSD |
| Flow rate (± 0.1 mL/min) | 1.4 | 0.6207 ± 0.0059 | 0.95 |
| 1.5 | 0.6455 ± 0.0024 | 0.38 | |
| 1.6 | 0.6545 ± 0.0029 | 0.44 | |
| Detection wavelength (± 2nm) | 214 | 0.6148 ± 0.0012 | 0.19 |
| 216 | 0.6602 ± 0.0029 | 0.42 | |
| 218 | 0.6347 ± 0.0023 | 0.37 | |
| Mobile phase composition ratio (± 2mL) | 60:40:00 | 0.6283 ± 0.0036 | 0.56 |
| 62:38:00 | 0.6602 ± 0.0028 | 0.42 | |
| 58:42:00 | 0.6328 ± 0.0062 | 0.99 |
Table 4: Robustness of developed and reference methods of the instrument in measuring the absorbance of Artemether Standard.
| Standard Concentration (mg/mL) | Peak Response |
|---|---|
| 0.25 | 0.686 |
| 0.2 | 0.582 |
| 0.15 | 0.483 |
| 0.1 | 0.367 |
| 0.05 | 0.287 |
| Mean | 0.481 |
| STDEV | 0.1604 |
| %RSD | 33.3389 |
Table 5: Linearity and range of the instrument in measuring the absorbance of Artemether Standard.
| Sample Code | Before degradation | Thermal | Photolysis | Acid | Base |
|---|---|---|---|---|---|
| A | 0.199 ± 0.09fgh | 0.537 ± 0.002g | 0.829 ± 0.010g | 0.047 ± 2.31k | 0.064 ± 2.10k |
| B | 0.209 ± 1.33h | 0.450 ± 0.001bc | 0.693 ± 0.002c | 0.074 ± 0.89d | 0.073 ± 0.50c |
| C | 0.164 ± 0.50abc | 0.494 ± 0.003f | 0.765 ± 0.020f | 0.077 ± 1.20f | 0.073 ± 0.75g |
| D | 0.175 ± 0.24bcde | 0.477 ± 0.001d | 0.751 ± 0.008f | 0.059 ± 1.45h | 0.058 ± 1.10i |
| E | 0.189 ± 1.24efgh | 0.623 ± 0.016h | 0.742 ± 0.010def | 0.032 ± 2.40k | 0.057 ± 0.95i |
| F | 0.182 ± 0.47cdef | 0.446 ± 0.021b | 0.724 ± 0.003de | 0.054 ± 1.30h | 0.076 ± 0.80d |
| G | 0.192 ± 4.84efgh | 0.466 ± 0.006cd | 0.649 ± 0.003b | 0.060 ± 0.90e | 0.063 ± 0.65cd |
| H | 0.187 ± 1.44defgh | 0.486 ± 0.008e | 0.760 ± 0.031f | 0.095 ± 0.40c | 0.078 ± 0.90e |
| I | 0.156 ± 2.71ab | 0.468 ± 0.002cd | 0.617 ± 0.017a | 0.077 ± 0.23b | 0.074 ± 0.30a |
| J | 0.188 ± 19.54abcd | 0.477 ± 0.001d | 0.745 ± 0.027def | 0.059 ± 0.94h | 0.072 ± 0.87f |
| K | 0.204 ± 15.57gh | 0.623 ± 0.016h | 0.752 ± 0.006f | 0.021 ± 2.50l | 0.058 ± 1.50k |
| L | 0.206 ± 0.99h | 0.446 ± 0.021b | 0.818 ± 0.002g | 0.053 ± 2.40j | 0.068 ± 0.50j |
| M | 0.150 ± 11.82a | 0.537 ± 0.002g | 0.721 ± 0.001d | 0.096 ± 0.10a | 0.061 ± 0.40b |
| N | 0.197 ± 0.45efgh | 0.415 ± 0.004a | 0.603 ± 0.002a | 0.072 ± 1.70g | 0.078 ± 0.62f |
| O | 0.182 ± 0.39cdef | 0.487 ± 0.003f | 0.746 ± 0.002ef | 0.047 ± 1.00i | 0.056 ± 0.63h |
Note: Values are Mean ± SD, Values with different superscript letter down the column are significantly different at p <0.05. Any two or more means having a common letter, are not significantly different at the 5% level of significance.
Table 6: Linearity and range of the instrument in measuring the absorbance of Artemether Standard.
| Sample Code | Control | Before degradation | Thermal | Photolysis | Acid | Base |
|---|---|---|---|---|---|---|
| A | - | 123.12 ± 0.09fgh | 47.05 ± 2.10g | 18.34 ± 0.65g | 71.72 ± 2.31k | 61.41 ± 2.10k |
| B | - | 129.32 ± 1.33h | 39.25 ± 1.70d | 15.34 ± 0.40cd | 39.32 ± 0.89d | 39.45 ± 0.50c |
| C | - | 101.42 ± 0.50abc | 46.43 ± 1.50fg | 19.72 ± 0.35h | 48.59 ± 1.20f | 51.49 ± 0.75g |
| D | - | 108.18 ± 0.24bcde | 43.59 ± 1.20e | 16.59 ± 0.50e | 55.25 ± 1.45h | 55.91 ± 1.10i |
| E | - | 117.29 ± 1.24efgh | 28.20 ± 0.85b | 16.48 ± 0.60e | 70.32 ± 2.40k | 55.50 ± 0.95i |
| F | - | 113.08 ± 0.47cdefg | 43.40 ± 1.30e | 16.04 ± 0.70de | 54.80 ± 1.30h | 41.66 ± 0.80d |
| G | - | 118.75 ± 4.84efgh | 32.41 ± 1.00c | 14.38 ± 0.40bc | 42.27 ± 0.90e | 40.36 ± 0.65cd |
| H | - | 115.94 ± 1.44defgh | 43.84 ± 2.00e | 16.84 ± 0.90e | 34.68 ± 0.40c | 45.06 ± 0.90e |
| I | - | 96.37 ± 2.71ab | 28.32 ± 0.90b | 13.67 ± 0.80b | 27.95 ± 0.23b | 29.89 ± 0.30a |
| J | - | 102.57 ± 19.54abcd | 42.87 ± 1.00e | 16.49 ± 0.55e | 54.53 ± 0.94h | 46.65 ± 0.87f |
| K | - | 126.66 ± 15.57gh | 36.49 ± 0.50d | 23.81 ± 0.70i | 85.51 ± 2.50l | 62.74 ± 1.50k |
| L | - | 127.34 ± 0.99h | 54.42 ± 3.00h | 17.83 ± 0.50fg | 66.79 ± 2.40j | 57.61 ± 0.50j |
| M | - | 92.82 ± 11.82a | 18.85 ± 0.77a | 0.39 ± 0.01a | 14.02 ± 0.10a | 34.85 ± 0.40b |
| N | - | 122.03 ± 0.45efgh | 53.41 ± 2.90h | 34.89 ± 0.88j | 51.28 ± 1.70g | 47.56 ± 0.62f |
| O | - | 112.56 ± 0.39cdef | 38.95 ± 1.45d | 13.43 ± 0.60b | 58.86 ± 1.00i | 53.24 ± 0.63h |
Note: Values are Mean ± SD, Values with different superscript letter down the column are significantly different at p <0.05. Any two or more means having a common letter, are not significantly different at the 5% level of significance.
Table 7: The percent content of drug substance of artemether injections sample before and after degradation.
System suitability test
The standard solution which was prepared as per the procedure and was injected six-times, injections response of the standard drug was used to determine the relative standard deviation in percentage (%RSD) which was 0.42 used to validate the consistency of the results obtained from the instrument.
Accuracy
The accuracy of an analytical method validation is the closeness of test results obtained by that method to the true value (Standard value) using known quantity of Artemether standard obtaining %RSD of these test to be 0.46, 0.71 and 0.68 respectively
Precision
The precision for repeatability of the instrument in the intra-day had a %RSD of 0.83 while the inter-days had a %RSD of 1.26
Robustness
The instrumental processes to remain unaffected by the deliberate variation in the method parameters with a range of %RSD of 0.19 to 0.99.
Linearity and range
The calibration curves constructed by plotting peak area against the respective concentrations recorded R^2=0.9976 and the regression equation of y=2.026 × +0.177
Limit of detection and limit of quantitation
Sensitivity of the proposed method was estimated in terms of Limit of Detection (LOD) at 0.53 and Limit of Quantitation (LOQ) at 1.61
Photolysis degradation
After the samples were exposed to light in a closed cardboard at 366 nm photolytic degradation may occur through isomerization, dimerization, cyclization, rearrangement, decarboxylation and haemolytic cleavage of X-C hetero bonds.
Samples (N) and (K) recorded 34.9% and 23.8% degradations the highest in the photolytic environment with 122.0% and 126.7% percent content before degradation.
Samples (C), (A), and (L) recorded 19.7%, 18.3%, and 17.8% degradation of the active pharmaceutical ingredient of the samples which was about 2% below the maximum acceptance criteria of 20% with 101.4%, 123.1% and 127.3% percent content of the active pharmaceutical ingredient of the sample respectively before degradation.
Samples (H), (D), (E), (J), and (F) recorded 16.8%, 16.6%, 16.5%, 16.5%, and 16.0% degradation of the active pharmaceutical ingredient of the samples which was 4% below the maximum acceptance criteria of 20% with 115.9%, 108.2%, 117.3%, 102.6% and 113.1% percent content before degradation of the samples respectively.
Sample (B), (G), (I), and (O) recorded 15.3%, 14.4% and 13.7%, 13.4% degradation of the active pharmaceutical ingredient of the samples which was about 6% below the maximum acceptance criteria of 20% with 129.3%, 118.8%, 96.4%, and 112.6% percent content before degradation of the samples respectively. The lowest degradation recorded in the photolytic environment was by sample (M) with 0.4% degradation of the active pharmaceutical ingredient of the sample which was 19.6% below the maximum acceptance criteria of 20% with 92.8% percent content before degradation of the sample as established by International Conference on Harmonization 2003. The trend of the photolytic degradations was: N>K>>C>A>L>H>D>E>J>F>B>G>I>O>M
Thermal degradation
Sample (L), (N), (A), and (C) recorded 54.4%, 53.4%, 47.1% and 46.4% degradation of the active pharmaceutical ingredient of the sample which was about 30% above the maximum acceptance criteria of 20% with 127.3%, 122.0%, 123.1%, and 101.4% percent content of the active pharmaceutical ingredient of the sample before degradation of the samples respectively in thermal environment.
Sample (H), (D), (F), (J), (B), (O), and (K) recorded 43.8%, 43.6%, 43.4%, 42.9%, 39.3%, 39.0% and 36.5% degradation of the active pharmaceutical ingredient of the sample which was 20% above the maximum acceptance criteria of 20% with 115.9%, 108.2%, 113.1%, 102.6%, 129.3%, 112.6%, and 126.7% percent content of active pharmaceutical ingredient of the samples before degradation respectively in the thermal environment.
Samples (G), (I), and (E) recorded 32.4%, 28.3%, and 28.2% degradation of the active pharmaceutical ingredient of the samples which was about 10% above the maximum acceptance criteria of 20% with 118.8%, 96.4%, and 117.3% percent content of the active pharmaceutical ingredient of the samples before degradation of the samples in the thermal environment.
Sample (M) recoded 18.9% degradation of the active pharmaceutical ingredient of the sample which was 2% below the maximum acceptance criteria of 20% with 92.8% percen content of active pharmaceutical ingredient before degradation of the sample in the thermal environment as established by International Conference on Harmonization 2003. The trend of the thermal degradation was: L>N>A>C>H>D>F>J>B>O>K>G>I>E>M
Acidic degradation
The exposure of the sample to acid degradation resulted in all the samples degrading beyond the 20% maximum acceptance limit for induced degradation as stipulated by the International Conference Harmonization. This is due to the cleavage of the peroxide bridge in the artemether molecule to form dihydroartemisinin molecules. The artemisinin derivatives act quickly and are eliminated quickly. The anti-parasitic activity of artemisinin and its derivative are related to the endoperoxide bridge in its structure.
Sample (K) had the highest degradation in acid with 85.5% of the sample degrading its active pharmaceutical ingredient in the acidic environment which was about 65% above the maximum acceptance criteria of 20%. This sample had a recorded percent content before degradation of 126.7% of the sample’s active pharmaceutical ingredient. Samples (A), (E) and (L) recorded 71.7%, 70.3%, and 66.9% degradations in the acidic environment respectively with a percent content of 123.1%, 117.3%, and 127.3% before degradation. Their resulting degradations were about 50% above the maximum acceptance criteria of 20%.
Samples (O), (D), (F), (J), (N) and (C) recorded degradation of 58.9%, 55.3%, 54.8%, 54.5%, 51.3%, and 48.6% of the drug substance with 112.6, 108.2, 113.1, 102.6, 122.0 and 101.4% percent content before they were subjected to acid degradation. This resulted in more than 30% degradation above the acceptable degradation limit. Samples (G), (B), (H), and (I) recorded 39.3%, 42.3%, 34.7%, and 28.0% degradations with percent content of 118.8%, 29.3%, 115.9%, and 96.4% before degradation which was about 40% above the maximum acceptance criteria of 20%.
Sample (M) had a degradation of 14% and percent content of 92.8% before degradation. Out of the fifteen samples analyzed sample (M) did not experience degradation above the 20% maximum acceptance criteria. Fourteen samples degrade above 20% maximum acceptance criteria with the highest degradation as established in the acidic environment based on the International Conference on Harmonization, 2003. This trend of degradation in the acidic medium agrees with studies of artemether carried out by Deepti Jain and Pawan Kumar Basniwal in 2013. The general trend of the observed for this degradation among the samples was: K>A>E>L>O>D>F>J>N>C>G>B>H>I>M.
Basic degradation
The degradation exposes the drug substance and the entire samples experience degradation above the 20% maximum acceptance criteria of its active content degraded under basic environmental condition. Sample (K) recorded the highest degradation in the basic environment with 62.7% degradation of the active pharmaceutical ingredient of the sample which recorded 126.7% percent content of the sample active pharmaceutical ingredient before exposure to basic environment. Following closely was sample (A) with a degradation of 61.4% of the active pharmaceutical ingredient of the sample with a recorded 123.1% percent content of the sample active pharmaceutical ingredient before exposure to the basic environment. These two samples had a degradation of more than 40% of the active pharmaceutical ingredient of the sample which is above the maximum acceptance criteria of 20% as established by international conference on harmonization.
Sample (L), (D), and (E) recorded 57.6%, 55.9% and 55.5% degradation of active pharmaceutical ingredient of the sample after exposure to basic environment with a 127.3, 117.3 and 108.2% respectively of active pharmaceutical ingredient before degradation. Their degradations were about 35% above the maximum acceptance criteria of 20%. Sample (O), (C), (N), (J), and (H) recorded 53.2%, 51.5%, 47.6%, 47.0% and 45.0% degradation of active pharmaceutical ingredient of the sample on exposure to the basic environment with a 112.6%, 101.4%, 122.0%, 102.6% and 115.9% percent content of active pharmaceutical ingredient of the sample before degradation respectively which is about 25% above the maximum acceptance criteria.
Samples (F), (G), and (B) recorded 41.7%, 40.4%, and 39.5% degradation of the active pharmaceutical ingredient of the sample which was 20% above the maximum acceptance criteria of 20% with 113.1%, 118.8%, and 129.3% percent content of Active pharmaceutical ingredient of the sample before degradation. Samples (M) and (I) recorded 34.9% and 29.9% degradation of Active pharmaceutical ingredient of the sample which was about 10% above the maximum acceptance criteria of 20% with 92.8%, and 96.4% percent content of Active pharmaceutical ingredient of the samples before degradation. These samples are the lowest recorded degradations in the basic environment as established by International Conference on harmonization 2003. This trend of degradation in the basic medium agrees with the studies of artemether carried by Deepti Jain and Pawan Kumar Basniwal in 2013. The general trend of the observed for this degradation among the samples was: K>A>L>D>E>O>C>N>J>H>E>G>B>M>I.
Conclusion
The quality of the artemether injection samples before degradation had shown that five out of the fifteen samples’ percent content recorded within the international pharmacopeia standard range of 90.0 to 110.0%. The whole samples recorded their highest stability in the photolytic environment with the exception of sample N and K recording instability in the photolytic environment at 34.4% and 23.8% degradation. All the samples recorded their highest degradation in the acidic environment with the exception of sample (M) recording stability in the acidic environment at 14.0% degradation. These records agree with the work of Deepti Jain and Pawan Kumar Basniwai.
References
- Cesar IC, Antonio G. Quantitation of artemether in pharmaceutical raw material and injections by HPLC. Brazilian Journal of Pharmaceutical Sciences. 2009;45:738-742.
- Cibulskis RE, Aregawi M, Williams R, Otten M, Dye C. Worldwide incidence of malaria in 2009: Estimates, time trends, and a critique of methods. PLOS Medicine. 2011;8. e1001142.
[Crossref] [Google Scholar] [PubMed]
- Menkin-Smith L, Winders WT. Plasmodium vivax Malaria. In: Stat Pearls Treasure Island (FL): Stat Pearls. 2024.
- International Conference on Harmonization. Guidance for industry, q2b validation of analytical procedures: Methodology. 1996.
- Iram F, Iram H, Iqbal A, Husain A. Forced degradation studies. J Anal Pharm Res. 2016;3:1
- Szepesi G, Gazdag M, Mihalyfi K. Selection of high-performance liquid chromatographic methods in pharmaceutical analysis: III. Method validation. Journal of Chromatography A. 1991;464:256-278.
[Crossref] [Google Scholar] [PubMed]
- Blassy M, Patel RD, Prajapati PN, Agrawal YK. Development of forced degradation and stability indicating studies of drugs-A review. J Pharm Anal.2014;4:159-165.
[Crossref] [Google Scholar] [PubMed]
- Jenke DR. Chromatographic method validation: A review of common practices and procedures II, J. Lig Chromatogr. 1996;19:737-757.
- R. Maheswaran. FDA perspectives: Scientific consideration of forced degradation studies in ANDA submissions. Pharm Technol. 2012;36:73-80.
- G. Ngwa. Forced degradation studies as an integral part of HPLC stability indicating method development. Drug Deliv Technol. 2010;56-59.
- Khushbu A, Thakor Dhara D. Bhavsar, Jagruti RT. A review article-development of food degradation and stability indicating studies for drug substance and drug product. International Journal of Research in Pharmacology & Pharmacotherapeutics. 2021;5:291-297.
- Singh S, Baskshi M. Guidance on conduct of stress tests to determine inherent stability of drugs, Pharm. Technol. 2000;24:1-14.
- Bakshi M, Singh S. Development of validated stability-indicating assay methods-critical review. J Pharm Biomed Anal. 2002;28:1011-1040.
[Crossref] [Google Scholar] [PubMed]
- Larsen B, Bundgaard H. Polymerization of penicillins: V. Separation, identification and quantitative determination of Antigenic polymerization products in Ampicilin Sodium preparations by high-performance liquid chromatography. J Chromatogr A. 1978;147, 143-150.
- Roma R. Temple. United States Pharmacopeia National Formulary USPNF. ISSUE 2. 2023.
- International Conference on Harmonization. Guidance for Industry. Q1A (R2): Stability testing of new drug substances and product. 2003.
- Jain D, Basniwal PK. Forced degradation profiling of artemether by validated stability indicating RP-HPLC-DAD method. Hacettepe University Journal of the Faculty of Pharmacy. 2013;33:41-58.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.