Serum Soluble Endoglin as Predictor of Non-severe Pre-eclampsia and Severe Pre-eclampsia in early Second Trimester of Pregnancy
Citation: Sachan R, et al. Serum Soluble Endoglin as Predictor of Non Severe Preeclampsia and Severe Preeclampsia in early Second Trimester of Pregnancy. Ann Med Health Sci Res. 2018;8:179-183
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Objective: To evaluate the role of serum soluble endoglin in prediction of non-severe preeclampsia and severe preeclampsia in early second trimester (13-20 weeks) of pregnancy. Material and Methods: Prospective case–control study was carried out over a period of one and half years. Total 543 pregnant women were recruited but 43 were lost to follow up. Out of 500 pregnant women, 34 developed non severe preeclampsia (Group 2) and 16 developed severe preeclampsia (Group 3), and 51 healthy normotensive pregnant women (Group 1) were selected as controls. Serum soluble endoglin levels were measured by Human Eng (endoglin) ELISA kit. Endoglin levels were estimated by enzyme linked immune-sorbent assay technique. Results: Prevalence of hypertensive disorders of pregnancy (HDP) was 10.2%. The mean sEng levels were significantly higher in non-severe preeclampsia and severe preeclampsia as compared to controls (p<0.001). The mean sEng level was significantly elevated, almost more than doubled from baseline value, after the development of preeclampsia in both groups, non-severe preeclampsia (6.95 ± 0.19 vs. 13.65 ± 0.32, p<0.001) and severe preeclampsia (7.99 ± 0.27 vs. 17.81 ± 0.52, p<0.001) as compared to baseline values. At cut-off value of sEng >6.10 ng/ml not only discriminate non severe preeclampsia from controls with 82.35% sensitivity, 88.24% specificity, but also predict severe preeclampsia means severity of disease with 93.75% sensitivity, 88.24% specificity. To discrimination between non severe and severe PE, at cut–off value of >7.76 ng/ml, sEng had 68.75% sensitivity, 85.29% specificity, positive predictive value 68.7% and negative predictive value 85.3%. Conclusion: Thus sEng can be used as a potential biomarker to predict preeclampsia as early as in second trimester (13-20 weeks) of pregnancy.
Introduction
The most common complication during pregnancy is hypertensive disorders complicating pregnancy (HDP). Severe preeclampsia is a leading cause of maternal and perinatal mortality and morbidity worldwide.
In India approximately 72,000 pregnant women die every year means nearly 200 deaths every day because of eclampsia and severe preeclampsia. Preeclampsia–eclampsia ranks second next to hemorrhage as a specific, direct cause of maternal death. In developing countries the risk of death due to preeclampsia or eclampsia is about 300 times more than that of developed countries. [1] Incidence of HDP in India is found to be 10.08%. National Eclampsia Registry data of over the past 3 years reported, out of 1,11,725 deliveries, 2,554 patients developed eclampsia. Word wide HDP complicates approximately 5–10% of pregnancies. [2] The incidence of eclampsia is 0.3% to 0.9% and it has a maternal mortality rate of 0.5%–10.0%. [3] In Africa and Asia 9% maternal deaths, were due to hypertensive diseases, whereas, in Latin America and the Caribbean, the figure is over 25%. [4]
Despite of intensive research, etiology of preeclampsia is exactly not known, various factors including placental ischemia, genetic predisposition, and immunological maladaptation might play important role in development of preeclampsia.
Several predictive markers including placental growth factor, soluble fms-like tyrosine kinase-1 (sFlt-1), plasma protein 13, and pregnancy-associated plasma protein-A (PAPP-A) have been evaluated, but none is recommended in routine clinical use. [5] Therefore it is very important to find out biochemical marker that could predict the development of preeclampsia few weeks early before the development of disease. Defect in angiogenesis during early pregnancies leads to incomplete remodelling of spiral arterioles thus leading to abnormal placental vascular development in Preeclampsia. Soluble anti-angiogenic factor serum soluble endoglin is secreted in excess by the placenta in the maternal circulation in preeclampsia. This might be responsible for endothelial dysfunction and clinical signs of preeclampsia. Endoglin is a homodimeric transmembrane glycoprotein which is accepted as co receptor for transforming growth factor beta (TGF β). [6] VEGF, placental growth factor, SFLT-1, contributes to endothelial cell dysfunction in maternal vasculature creating imbalance of angiogenic and antiangiogenic factors in circulation. sEng acts by antagonizing an angiogenic & vasodilator molecule known as transforming growth factor beta-1, which is important in angiogenesis, thus inhibiting eNOS and it keeps the lining of blood vessels healthy. Due to defective angiogenesis and vasoconstriction, cell lining the blood vessels begin to sicken & die, blood pressure increases and protein leak from blood vessels into the tissues & urine. [7] Thus by this study our aim is to establish the role of serum soluble endoglin in prediction of non-severe preeclampsia and severe preeclampsia in early second trimester (13-20 weeks) of pregnancy.
Material and Methods
This prospective case–control study was carried out in the department of Obstetrics and Gynaecology, King George’s Medical University, Lucknow from March 2015 to August 2016. After informed consent and ethical clearance from institutional ethic committee, total 543 pregnant women enrolled for the study from those who attended the antenatal clinic at 13 -20th weeks of gestation. 43 women lost follow up. Thus 500 pregnant women were followed till six weeks postpartum for development of disease. Women with multifetal pregnancy, chronic hypertension, diabetes, chronic liver disease, chronic kidney disease, collagen vascular diseases, major fetal anomaly, cardiovascular diseases, and women with history of smoking or alcohol intake were excluded from the study. Out of 500 pregnant women, 34 were developed mild preeclampsia (Group 2) and 16 developed severe preeclampsia (Group 3), and 51 healthy normotensive pregnant women (Group 1) selected as controls, after systematic randomization from those who did not developed disease during follow up period. Non severe preeclampsia and severe preeclampsia was defined as per American College of Obstetrics and Gynaecology (ACOG) 2013b guidelines. [8] Only one woman developed eclampsia, so this was excluded during final analysis.
In present study 5 ml venous blood sample was collected twice, sEng levels were measured twice, first at the time of recruitment (13-20 weeks) and second time after the development of disease (preeclampsia and eclampsia). Samples centrifuged at 6000 rpm and kept at -20ºC until assay. Serum soluble endoglin level was measured by enzyme linked immunosorbent assay technique as per producer protocol, by commercially available Human Eng (endoglin) ELISA kit, manufactured by Elab Science Inc. Sensitivity for minimum detectable dose of Human ENG is 0.1 ng/ml. detection range-0.16-10 ng/ml, kit recognizes natural and recombinant human ENG. No significant cross reactivity or interference between human ENG and analogues was observed.
Modified ACOG 2013b, diagnostic criteria for non-severe preeclampsia is defined as BP>140/90 mmHg after 20 weeks of pregnancy in previously normotensive women with Proteinuria ≥ 300 mg/24 hr or Protein/creatinine ratio ≥0.3 with mildly elevated liver enzymes. Severe preeclampsia is defined as BP>160/110mmHg with platelets counts <100,000/ μl, creatinine >1.1 mg/dl or doubling of baseline, SGOT/SGPT levels twice than normal with headaches, visual disturbances and when preeclampsia is associated with convulsions then it is called eclampsia. [8]
During this study period after admission the demographic data of the patients was recorded. BP was measured three times per day and labour strictly monitored. All patients were given antihypertensive drugs however, mild preeclampsia cases, were treated by Methyldopa and in severe preeclampsia, the patients were treated with Labetalol. Methyldopa is a centrally acting US Food and Drug Administration category B drug considered safe for the mother and foetus. Labetalol, a Food and Drug Administration category C drug was given in cases of severe preeclampsia and eclampsia.
Magnesium sulfate, according to the Pritchard regimen, was administered to control convulsions in patients with eclampsia, along with fluid replacement in addition, patients with eclampsia were intensively monitored. Injection dexamethasone 6 mg intramuscular 12 hourly a total 4 dose was administered to achieved the lung maturity of foetus.
Statistical Analysis
All statistical evaluations of the data were done with Statistical Program for Social Sciences (SPSS, Version 17) software. The variables were stated as mean ± standard deviation. In the comparison of the data between the patient and control groups, the ANOVA tests were used. A p value of <0.05 was accepted as statistically significant. For prediction of preeclampsia ROC curve analysis was done.
Results
Out of the total 500 recruited pregnant women, 34 (6.8%) developed non severe preeclampsia, 16 (3.2%) developed severe preeclampsia and only one woman developed eclampsia (0.2%) thus the prevalence of hypertensive disorder of pregnancy (HDP) was 10.2%.
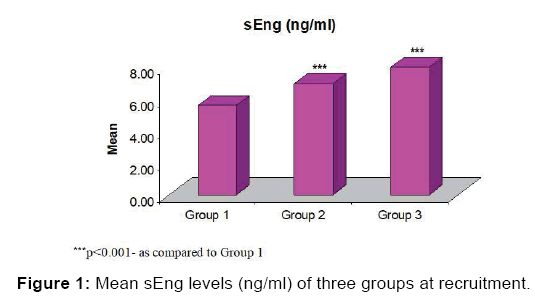
The demographic characteristics of three groups at presentation were similar except BMI. The mean BMI of Group 3 was significantly (p<0.024) different and higher (25.13 ± 0.96) than Group 1 (22.10 ± 0.52). Mean age of Group 2 and group 3 almost similar [Table 1]. At recruitment serum soluble endoglin (sEng) level of three groups after comparison by ANOVA test showed, mean sEng levels were significantly higher in Group 2 (non-severe preeclampsia) and Group 3 (severe preeclampsia) as compared to controls (p<0.001) [Table 2].
| Demographic characteristics | Group 1 (controls) (n=51) (%) |
Group 2 (NSPE) (n=34) (%) |
Group 3 (SPE) (n=16) (%) |
F/χ2 value |
p value |
|---|---|---|---|---|---|
| Age (yrs) | 25.90 ± 0.57 | 25.56 ± 0.58 | 25.13 ± 0.97 | 0.27 | 0.765 |
| BMI (kg/m2) | 22.10 ± 0.52 | 23.06 ± 0.69 | 25.13 ± 0.96 | 3.86 | 0.024 |
| SES Upper Middle Lower |
3 (5.9) 33 (64.7) 15 (29.4) |
2 (5.9) 23 (67.6) 9 (26.5) |
1 (6.3) 9 (56.3) 6 (37.5) |
0.67 | 0.955 |
| Parity Primi Gravida 2 Gravida 3 Gravida 4 Gravida 5 |
20 (39.2) 13 (25.5) 11 (21.6) 5 (9.8) 2 (3.9) |
21 (61.8) 7 (20.6) 5 (14.7) 1 (2.9) 0 (0.0) |
11 (68.8) 1 (6.3) 1 (6.3) 2 (12.5) 1 (6.3) |
10.58 | 0.227 |
| Blood group A B AB O |
13 (25.5) 21 (41.2) 6 (11.8) 11 (21.6) |
8 (23.5) 19 (55.9) 2 (5.9) 5 (14.7) |
3 (18.8) 10 (62.5) 1 (6.3) 2 (12.5) |
3.56 | 0.736 |
| Rh Positive Negative |
42 (82.4) 9 (17.6) |
28 (82.4) 6 (17.6) |
14 (87.5) 2 (12.5) |
0.26 | 0.880 |
| NSPE-Non Severe Preeclampsia, SPE-Severe Preeclampsia, SES-Socio-Economic Status | |||||
Table 1: Demographic characteristics (Mean ± SD) of three groups (Cases and controls).
| Group 1 (controls) (n=51) (Mean ± SE) |
Group 2 (NSPE) (n=34) ) (Mean ± SE) |
Group 3 (SPE) (n=16) (Mean ± SE) |
F value |
p value |
|---|---|---|---|---|
| 5.60 ± 0.19 | 6.95 ± 0.19 | 7.99 ± 0.27 | 26.37 | <0.001 |
Table 2: sEng levels (ng/ml) of three groups at recruitment.
After Tukey test analysis it was observed that the mean sEng levels were higher in both group 2 and group 3. Moreover, it was also found significantly (p<0.05) higher in Group 3 as compared to Group 2 [Table 3, Figure 1]. The sEng levels in cases (Group 2 and Group 3) were compared before and after development of HDP. Paired t-test analysis showed significantly elevated levels (almost twice) after development of HDP in both groups, Group 2 (6.95 ± 0.19 vs. 13.65 ± 0.32, p<0.001) and Group 3 (7.99 ± 0.27 vs. 17.81 ± 0.52, p<0.001) [Table 4].
| Comparisons | p-value |
|---|---|
| Group 1 vs. Group 2 | <0.001 |
| Group 1 vs. Group 3 | <0.001 |
| Group 2 vs. Group 3 | 0.021 |
Table 3: Comparison (p value) of mean sEng levels (ng/ml) between groups at recruitment by Tukey test.
| Group | Before (Recruitment) (Mean ± SE) | After (Development of HDP) (Mean ± SE) | t value | p value |
|---|---|---|---|---|
| Group 2 (NSPE) (n=34) | 6.95 ± 0.19 | 13.65 ± 0.32 | 18.49 | <0.001 |
| Group 3 (SPE) (n=16) | 7.99 ± 0.27 | 17.81 ± 0.52 | 18.86 | <0.001 |
| NSPE-Non Severe Pre-eclampsia, SPE-Severe Preeclampsia, HDP-Hypertensive Disorders of Pregnancy Means Non Severe and Severe Preeclampsia | ||||
Table 4: sEng levels (ng/ml) of two groups, Group 2 and Group 3 before and after development of HDP.
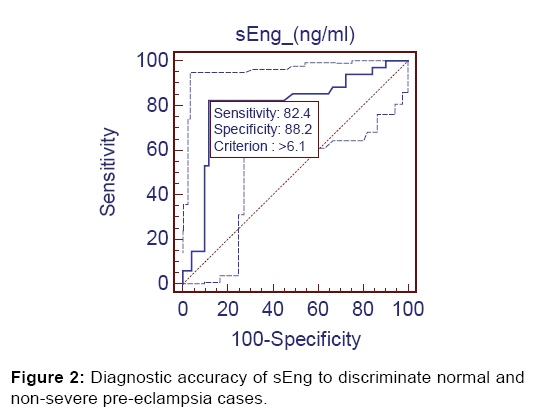
After Receiver Operating Characteristics (ROC) analysis significant diagnostic accuracy of sEng was observed. At cutoff value of >6.10 ng/ml (AUC=0.797), it discriminated the non-severe preeclampsia (Group 2) from controls (Group 1) with 82.4% sensitivity (95% CI=65.5-93.2), 88.24% specificity (95% CI=76.1-95.5), 82.4% positive predictive value and 88.2% negative predictive value [Figure 2].
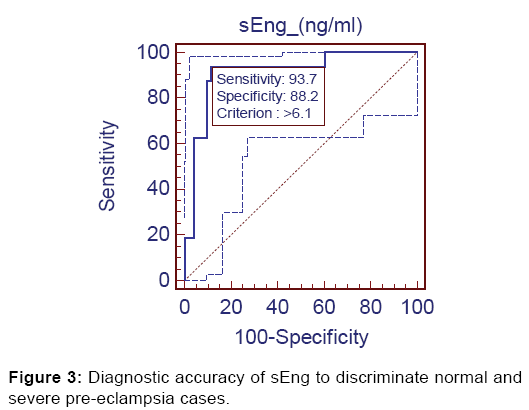
Further, significant diagnostic accuracy (AUC=0.913, p<0.001) was found when evaluated between Group 1 and Group 3. At the same cut-off value of >6.10 ng/ml it discriminated the severe preeclampsia from controls with 93.75% sensitivity (95% CI=69.7-99.0), 88.24% specificity (95% CI=76.1-95.5), 71.4% positive predictive value and 97.8% negative predictive value [Figure 3].
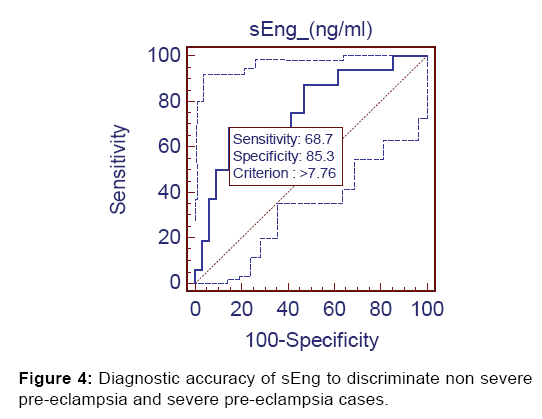
Lastly, at cut-off value of >7.76 ng/ml sEng discriminated non severe preeclampsia from severe preeclampsia with 68.75% sensitivity, 85.3% specificity, 68.7% positive predictive value and 85.3% negative predictive value [Figure 4].
Discussion
According to the ACOG, Task Force on hypertension, [8] preeclampsia is a progressive disease, progressing at different rates in different women and appropriate care requires. Thus need of frequent re-evaluation for severe features of the disease are mandatory. Prediction of the disease in early stage is very important, to identify before it turn to severe preeclampsia. Preeclampsia is a major contributor of maternal and neonatal morbidity and mortality. It can complicate up to 10% of pregnancies in developing countries. [3]
The exact pathophysiology of preeclampsia is unknown but generalized endothelial dysfunction with systemic inflammatory response (SIRS)is thought to be the final common pathway that leads to maternal signs of preeclampsia. [9] There are many proangiogenic and anti-angiogenic substances involved in placental vascular development. Various authors have described the role of these substances for diagnosis of preeclampsia & results are encouraging, yet till date no marker has been proved highly efficacious for the same.
In our study the prevalence of preeclampsia was 10.2% and prevalence of eclampsia was 0.2%. However Levine et al. reported 25.78% prevalence of HDP in their study. [10]
In present study mean age of Group 2 and group 3 was 25.56 ± 0.58 and 25.13 ± 0.97 respectively. Among cases most of the women belonged to middle socio-economic status (SES) and were primigravida. Various authors also reported Young age and primigravida are proven risk factors for the development of preeclampsia. [11-13] In our study no maternal death was reported this might be due to better antenatal care and only one patient developed eclampsia, this indicate if we provide good antenatal care incidence of eclampsia and maternal deaths definitely will be low. The maternal mortality rate of 17%–18% reported in patients with eclampsia in previous two old studies. [14,15] In our previous study four maternal deaths were reported and all in the eclampsia group, comprising 8.89%and overall maternal mortality rate was 2.8%. [16]
In the present study serum soluble endoglin (sEng) level of three groups at recruitment was significantly different (p<0.001). The mean levels were significantly higher in both Group 2 (non-severe preeclampsia) and Group 3 (severe preeclamsia) as compared to controls (5.60 ± 0.19 ng/ml) (p<0.001). Moreover, it was also found significantly higher in Group 3 as compared to Group 2 (p<0.05). The highest value of serum sEng was found in patient who developed eclampsia (19.1 ng/ ml), followed by severe preeclampsia (17.81 ± 0.52 ng/ml) than non-severe preeclampsia (13.65 ± 0.32 ng/ml). Thus sEng can be used as a potential biomarker to predict preeclampsia in early second trimester (13-20 weeks). Almost similar trend was observed by the Hamdy et al., a significant increase in serum sEng level in patients with severe preeclampsia comparison to mild preeclampsia (17.87 ± 2.11 ng/ml vs. 12.03 ± 1.7 ng/ml, respectively, (P<0.01). Value of serum sEng was 5.83 ± 0.81 ng/ml in patients without preeclampsia (P<0.01). [11] Gaber et al. also reported similar rising trend of sEng in their study, the median value of 3.1 ng/ml in controls, 6.2 ng/ml in cases with gestational hypertension and 9 ng/ml in cases who developed preeclampsia. The level of sEng was higher in preeclampsia as compared to controls (P <0.0005). [13]
After ROC curve analysis for prediction of non-severe preeclampsia cut-off value of sEng was >6.10 ng/ml, it had 82.4% sensitivity, 88.24% specificity, 82.4% positive predictive value and 88.2% negative predictive value. For prediction of severe preeclampsia at same cut off value, it had 93.75% sensitivity, 88.24% specificity, 71.4% positive predictive value and 97.8% negative predictive value. Lastly, the sEng at cutoff value of >7.76 ng/ml discriminating non severe and severe preeclampsia with 68.75% sensitivity, 85.3% specificity, 68.7% positive predictive value and 85.3% negative predictive value. Another study reported at the cut off values of 8.35 ng/ml, and 15.25 ng/ml optimally identified the patients with risk to develop preeclampsia and severe preeclampsia respectively. At this cut off value, the sensitivity was 100% and specificity was 100%. [11] One study reported the ability of this marker to differentiate preeclamptics from normal pregnancies at a cut-off value of 7 ng/ ml, sensitivity was 94.4%, specificity was 87.5% and accuracy was 89.5%. [13] At a cut-off value of 7 ng/ml, estimated odds ratio clearly emphasized that increasing sEng plasma level is a high risk for developing pre-eclampsia (OR = 119, P<0.0005). It seems that sEng inhibits the formation of capillaries in vitro and induces vascular permeability, correlating with severity of pre-eclampsia. [17] Rana et al. reported sEng level at 11–13 weeks gestation was 6.9 ± 0.3 ng/ml versus 6.6 ± 0.2 ng/ml, in preeclampsia versus normotensive controls respectively (P=0.37). At 17–20 weeks of gestation it was 6.4 ± 0.4 ng/ml versus 5.2 ± 0.1, P<0.01 (13). [18] Stepan et al. recently reported that using the combination of sFlt1 and sEng in second trimester was able to predict early-onset preeclampsia in women with abnormal uterine artery Doppler findings with 100% sensitivity and 93% specificity. [19] Salahuddin et al. observed that sEng had sensitivity and specificity of 90% and 95% for differentiating normal from pre-eclamptic pregnancies. [20] Sharon E Maynard et al. also concluded that sEng is higher in high-risk women; serum sEng is increased prior to onset of preeclampsia. [21]
Jonathan Lai et al. found that the median sEng MoM at 30-33 weeks was significantly higher in the PE group (1.39, IQR 0.94- 2.18) than in the controls (0.95, IQR 0.77-1.19), but at 11-13 weeks there was no significant difference between the groups. [22]
All of the above observations indicate that serum sEng has an important role in prediction of preeclampsia because it has high sensitivity, specificity and high negative predictive value. sEng is also useful in prediction of the severity of preeclampsia. Serum soluble endoglin (sEng) has been shown to be elevated in the serum of pre-eclamptic women several weeks before the onset of clinical signs of preeclampsia so it might interfere with endothelial cells function. [23] Thus sEng can be used as a potential biomarker for prediction of non-severe preeclampsia and severe preeclampsia in high risk pregnant women in early second trimester (13-20 weeks).
Limitations
Main limitation of our study is small sample size, evaluation of sEng as a candidate marker for prediction of preeclampsia requires larger studies. Thus prospective longitudinal studies in different populations are suggested to elucidate sEng as a diagnostic biomarker in preeclampsia patients.
Conclusion
Our data showed the usefulness of sEng as a potential marker with high accuracy for diagnosis of preeclampsia and its severity. Serum sEng levels can be used as an emerging biochemical marker for the prediction of preeclampsia or in other word this could help to improve pregnancy outcome in such type of patients, may allow identification of patient at risk for adverse outcome necessitating timely transfer to tertiary care centre, and administration of steroids, thus reducing unnecessary admission and intervention.
Conflict of Interest
I declare there is no conflict of interest
REFERENCES
- Engender Health. Balancing the scales: Expanding treatment for pregnant women with life-threatening hypertensive conditions in developing countries. A report on barriers and solutions to treat preeclampsia and eclampsia. New York, Engender Health, 2007.
- Zenebe W, Hailemariam S, Mirkuzie W. Hypertensive disorders of pregnancy in Jimma University specialized hospital. Ethiop J Health Sci. 2011;21:147–154.
- Sibai BM, Sarinoglu C, Mercer BM. Eclampsia. VII. Pregnancy out-come after eclampsia and long-term prognosis. Am J Obstet Gynecol. 1992;166:1757-1761.
- Khan KS, Wojdyla D, Say L, Gülmezoglu AM, Van Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet. 2006;367:1066-1074.
- Bellamy L, Casas JP, Hingorani AD, Williams DJ. Preclampsia and risk of cardiovascular disease and cancer in later life; systematic review and metaanalysis. BMJ. 2007;335:974.
- Goumans MJ, Valdimarsdottir G, Itoh S, Rosendahl A, Sideras P, Ten Dijke P. Balancing the activation state of the endothelium via two distinct TGF- beta type I receptors. EMBO J 2002;21:1743-1753.
- Parra Cordert M. Rodrigo R, Bajra P. Predictive of early and late preeclampsia from maternal characteristics uterine artery Doppler and markers of vasculogenesis during first trimester of pregnancy. Ultrasound and obstetrics and gynaecology. 2013;41:538-544.
- Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of ACOG Taskforce on hypertension in pregnancy 2013;122:1122-1131
- Nawara MH, Mohamed W, Marwa G, Nagwa T. Maternal serum soluble endoglin in patients with preeclampsia and gestational hypertension and its relation to Doppler study of the fetomaternal Circulation Med. J. Cairo Univ. 2010;78:117-121.
- Levine RJ, Lam C, Qian C, Yu KF, Maynard SE, Sachs BP, et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med. 2006;355:992-1005.
- EL-Said MH, Mohammed NE, Eldin El-Ashmawi HS, Ramzy Saad G. Role of serum soluble endoglin in patients with preeclampsia. Journal of Applied Sciences Research, 2013;9:1249-1255
- Duhan N, Sharma D, Garg N, Dahiya K, Sirohiwal D. Comparative evaluation of serum soluble endoglin level in preeclampsia and normotensive pregnant women. J Physiol Pathophysiol. 2011;2:47-51.
- Gaber K, Hamdy E, Hanafy A. Soluble endoglin as a new marker for prediction of pre-eclampsia in early pregnancy. Middle East Fertility Society Journal. 2010;15:42-46.
- Aali BS, Ghafoorian J, Mohamad-Alizadeh S. Severe preeclampsia and eclampsia in Kerman, Iran: complications and outcomes. Med Sci Monit. 2004;10:CR163-CR167.
- Sibai BM, Ramadan MK. Acute renal failure in pregnancies complicated by hemolysis, elevated liver enzymes, and low platelets. Am J Obstet Gynecol. 1993;168:1682-1687.
- Sachan R, Patel ML, Sachan P, Gaurav A, Singh M, Bansal B. Outcomes in hypertensive disorders of pregnancy in the North Indian population. International Journal of Women Health. 2013;5:101-108.
- Many A, Hubel CA, Fisher SJ, Roberts JM, Zhou Y. Invasive cytotrophoblasts manifest evidence of oxidative stress in preeclampsia. Am J Pathol 2000;156:321-331.
- Rana S, Cerdeira AS, Wenger J, Salahuddin S, Lim KH, Ralston SJ, et al. Plasma concentrations of soluble endoglin versus standard evaluation in patients with suspected preeclampsia. PLoS One. 2012;7:e48259.
- Stepan H, Unversucht A, Wessel N, Faber R. Predictive value of maternal angiogenic factors in second trimester pregnancies with abnormal uterine perfusion. Hypertension 2007;49:818-824.
- Salahuddin S, Lee Y, Vadnais M. Diagnostic utility of soluble FMS-like tyrosine kinase 1 and soluble endoglin in hypertensive diseases of pregnancy. Am J Obstet Gynecol. 2007;197:e1-e6.
- Maynard SE, Karumanchi SA. Angiogenic factors and preeclampsia. Semin Nephrol. 2011;31:33-46.
- Lai J, Syngelaki A, Poon LC, Nucci M, Nicolaides KH. Maternal serum soluble endoglin at 30-33 weeks in the prediction of preeclampsia. Fetal Diagn Ther. 2013;33:149-155.
- Gregory A. Structural and functional characteristics of a soluble form of endoglin in the context of preeclampsia. Graduate Department of Immunology, University of Toronto, Canada. 2011;1-94.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.